Cav1.2
Calcium channel, voltage-dependent, L type, alpha 1C subunit (also known as Cav1.2) is a protein that in humans is encoded by the CACNA1C gene.[5] Cav1.2 is a subunit of L-type voltage-dependent calcium channel.[6]
Structure and function
This gene encodes an alpha-1 subunit of a voltage-dependent calcium channel. Calcium channels mediate the influx of calcium ions (Ca2+) into the cell upon membrane polarization (see membrane potential and calcium in biology).[7]
The alpha-1 subunit consists of 24 transmembrane segments and forms the pore through which ions pass into the cell. The calcium channel consists of a complex of alpha-1, alpha-2/delta and beta subunits in a 1:1:1 ratio. The S3-S4 linkers of Cav1.2 determine the gating phenotype and modulated gating kinetics of the channel.[8] Cav1.2 is widely expressed in the smooth muscle, pancreatic cells, fibroblasts, and neurons.[9][10] However, it is particularly important and well known for its expression in the heart where it mediates L-type currents, which causes calcium-induced calcium release from the ER Stores via ryanodine receptors. It depolarizes at -30mV and helps define the shape of the action potential in cardiac and smooth muscle.[8] The protein encoded by this gene binds to and is inhibited by dihydropyridine.[11] In the arteries of the brain, high levels of calcium in mitochondria elevates activity of nuclear factor kappa B NF-κB and transcription of CACNA1c and functional Cav1.2 expression increases.[12] Cav1.2 also regulates levels of osteoprotegerin.[13]
Regulation
The activity of CaV1.2 channels is tightly regulated by the Ca2+ signals they produce. An increase in intracellular Ca2+ concentration implicated in Cav1.2 facilitation, a form of positive feedback called Ca2+-dependent facilitation, that amplifies Ca2+ influx. In addition, increasing influx intracellular Ca2+ concentration has implicated to exert the opposite effect Ca2+ dependent inactivation.[15] These activation and inactivation mechanisms both involve Ca2+ binding to calmodulin (CaM) in the IQ domain in the C-terminal tail of these channels.[16] Cav1.2 channels are arranged in cluster of eight, on average, in the cell membrane. When calcium ions bind to calmodulin, which in turn binds to a Cav1.2 channel, it allows the Cav1.2 channels within a cluster to interact with each other.[17] This results in channels working cooperatively when they open at the same time to allow more calcium ions to enter and then close together to allow the cell to relax.[17]

Clinical significance
Mutation in the CACNA1C gene, the single-nucleotide polymorphism located in the third intron of the Cav1.2 gene,[18] are associated with a variant of Long QT syndrome called Timothy's syndrome[19] and more broadly with other CACNA1C-related disorders,[19] and also with Brugada syndrome.[20] Large-scale genetic analyses have shown the possibility that CACNA1C is associated with bipolar disorder[21] and subsequently also with schizophrenia.[22][23][24] Also, a CACNA1C risk allele has been associated to a disruption in brain connectivity in patients with bipolar disorder, while not or only to a minor degree, in their unaffected relatives or healthy controls.[25].In a first study in Indian population, the Schizophrenia associated Genome-wide association study (GWAS) SNP was found not to be associated with the disease. Furthermore, the main effect of rs1006737 was found to be associated with spatial abilityefficiency scores. Subjects with genotypes carrying the risk allele of rs1006737 (G/A and A/A) were found to have higher spatial abilityefficiency scores as compared to those with the G/G genotype. While in healthy controls those with G/A and A/A genotypes were found to have higher spatial memoryprocessing speed scores than those with G/G genotypes, the former had lower scores than the latter in schizophrenia subjects. In the same study the genotypes with the risk allele of rs1006737 namely A/A was associated with a significantly lower Align rank transformed Abnormal and involuntary movement scale(AIMS) scores of Tardive dyskinesia(TD).[26]
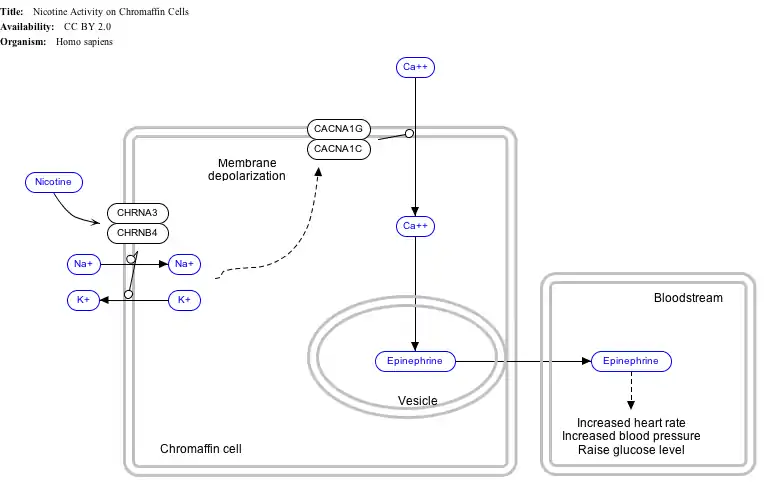
Interactive pathway map
Click on genes, proteins and metabolites below to link to respective Wikipedia articles. [§ 1]
- The interactive pathway map can be edited at WikiPathways: "NicotineActivityonChromaffinCells_WP1603".
References
- ENSG00000285479 GRCh38: Ensembl release 89: ENSG00000151067, ENSG00000285479 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000051331 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- Lacerda AE, Kim HS, Ruth P, Perez-Reyes E, Flockerzi V, Hofmann F, Birnbaumer L, Brown AM (Aug 1991). "Normalization of current kinetics by interaction between the alpha 1 and beta subunits of the skeletal muscle dihydropyridine-sensitive Ca2+ channel". Nature. 352 (6335): 527–30. Bibcode:1991Natur.352..527L. doi:10.1038/352527a0. PMID 1650913. S2CID 4246540.
- Catterall WA, Perez-Reyes E, Snutch TP, Striessnig J (Dec 2005). "International Union of Pharmacology. XLVIII. Nomenclature and structure-function relationships of voltage-gated calcium channels". Pharmacological Reviews. 57 (4): 411–25. doi:10.1124/pr.57.4.5. PMID 16382099. S2CID 10386627.
- Shaw RM, Colecraft HM (May 2013). "L-type calcium channel targeting and local signalling in cardiac myocytes". Cardiovascular Research. 98 (2): 177–86. doi:10.1093/cvr/cvt021. PMC 3633156. PMID 23417040.
- Lipscombe D, Helton TD, Xu W (Nov 2004). "L-type calcium channels: the low down". Journal of Neurophysiology. 92 (5): 2633–41. doi:10.1152/jn.00486.2004. PMID 15486420. S2CID 52887174.
- Christel C, Lee A (Aug 2012). "Ca2+-dependent modulation of voltage-gated Ca2+ channels". Biochimica et Biophysica Acta (BBA) - General Subjects. 1820 (8): 1243–52. doi:10.1016/j.bbagen.2011.12.012. PMC 3345169. PMID 22223119.
- Berger SM, Bartsch D (Aug 2014). "The role of L-type voltage-gated calcium channels Cav1.2 and Cav1.3 in normal and pathological brain function". Cell and Tissue Research. 357 (2): 463–76. doi:10.1007/s00441-014-1936-3. PMID 24996399. S2CID 15914718.
- "Entrez Gene: voltage-dependent, L type, alpha 1C subunit".
- Narayanan D, Xi Q, Pfeffer LM, Jaggar JH (Sep 2010). "Mitochondria control functional CaV1.2 expression in smooth muscle cells of cerebral arteries". Circulation Research. 107 (5): 631–41. doi:10.1161/CIRCRESAHA.110.224345. PMC 3050675. PMID 20616314.
- Bergh JJ, Xu Y, Farach-Carson MC (Jan 2004). "Osteoprotegerin expression and secretion are regulated by calcium influx through the L-type voltage-sensitive calcium channel". Endocrinology. 145 (1): 426–36. doi:10.1210/en.2003-0319. PMID 14525906.
- Cahalan MD (Oct 2010). "Cell biology. How to STIMulate calcium channels". Science. 330 (6000): 43–4. doi:10.1126/science.1196348. PMC 3133971. PMID 20929798.
- Isaev D, Solt K, Gurtovaya O, Reeves JP, Shirokov R (May 2004). "Modulation of the voltage sensor of L-type Ca2+ channels by intracellular Ca2+". The Journal of General Physiology. 123 (5): 555–71. doi:10.1085/jgp.200308876. PMC 2234499. PMID 15111645.
- Kim EY, Rumpf CH, Van Petegem F, Arant RJ, Findeisen F, Cooley ES, Isacoff EY, Minor DL (Dec 2010). "Multiple C-terminal tail Ca(2+)/CaMs regulate Ca(V)1.2 function but do not mediate channel dimerization". The EMBO Journal. 29 (23): 3924–38. doi:10.1038/emboj.2010.260. PMC 3020648. PMID 20953164.
- Dixon RE, Moreno CM, Yuan C, Opitz-Araya X, Binder MD, Navedo MF, Santana LF (2015). "Graded Ca²⁺/calmodulin-dependent coupling of voltage-gated CaV1.2 channels". eLife. 4. doi:10.7554/eLife.05608. PMC 4360655. PMID 25714924.
- Imbrici P, Camerino DC, Tricarico D (2013-05-07). "Major channels involved in neuropsychiatric disorders and therapeutic perspectives". Frontiers in Genetics. 4: 76. doi:10.3389/fgene.2013.00076. PMC 3646240. PMID 23675382.
- Napolitano C, Timothy KW, Bloise R, Priori SG (1993). Adam MP, Everman DB, Mirzaa GM, Pagon RA, Wallace SE, Bean LJ, Gripp KW, Amemiya A (eds.). CACNA1C-Related Disorders. PMID 20301577. Retrieved 2022-12-12.
{{cite book}}:|work=ignored (help) - Hedley PL, Jørgensen P, Schlamowitz S, Moolman-Smook J, Kanters JK, Corfield VA, Christiansen M (Sep 2009). "The genetic basis of Brugada syndrome: a mutation update". Human Mutation. 30 (9): 1256–66. doi:10.1002/humu.21066. PMID 19606473. S2CID 25207473.
- Ferreira MA, O'Donovan MC, Meng YA, Jones IR, Ruderfer DM, Jones L, et al. (Sep 2008). "Collaborative genome-wide association analysis supports a role for ANK3 and CACNA1C in bipolar disorder". Nature Genetics. 40 (9): 1056–8. doi:10.1038/ng.209. PMC 2703780. PMID 18711365.
- "Channeling Mental Illness: GWAS Links Ion Channels, Bipolar Disorder". Schizophrenia Research Forum. Archived from the original on 2010-12-18.
- Green EK, Grozeva D, Jones I, Jones L, Kirov G, Caesar S, Gordon-Smith K, Fraser C, Forty L, Russell E, Hamshere ML, Moskvina V, Nikolov I, Farmer A, McGuffin P, Holmans PA, Owen MJ, O'Donovan MC, Craddock N (Oct 2010). "The bipolar disorder risk allele at CACNA1C also confers risk of recurrent major depression and of schizophrenia". Molecular Psychiatry. 15 (10): 1016–22. doi:10.1038/mp.2009.49. PMC 3011210. PMID 19621016.
- Curtis D, Vine AE, McQuillin A, Bass NJ, Pereira A, Kandaswamy R, Lawrence J, Anjorin A, Choudhury K, Datta SR, Puri V, Krasucki R, Pimm J, Thirumalai S, Quested D, Gurling HM (Feb 2011). "Case-case genome-wide association analysis shows markers differentially associated with schizophrenia and bipolar disorder and implicates calcium channel genes". Psychiatric Genetics. 21 (1): 1–4. doi:10.1097/YPG.0b013e3283413382. PMC 3024533. PMID 21057379.
- Schizophrenia Working Group of the Psychiatric Genomics Consortium (2014-07-24). "Biological insights from 108 schizophrenia-associated genetic loci". Nature. 511 (7510): 421–427. Bibcode:2014Natur.511..421S. doi:10.1038/nature13595. ISSN 1476-4687. PMC 4112379. PMID 25056061.
- Radua J, Surguladze SA, Marshall N, Walshe M, Bramon E, Collier DA, Prata DP, Murray RM, McDonald C (May 2013). "The impact of CACNA1C allelic variation on effective connectivity during emotional processing in bipolar disorder". Molecular Psychiatry. 18 (5): 526–7. doi:10.1038/mp.2012.61. PMID 22614292.
- Punchaichira TJ, Kukshal P, Bhatia T, Deshpande SN (2023). "Effect of rs1108580 of DBH and rs1006737 of CACNA1C on Cognition and Tardive Dyskinesia in a North Indian Schizophrenia Cohort". Molecular Neurobiology. doi:10.1007/s12035-023-03496-4. PMID 37493923.
Further reading
- Kempton MJ, Ruberto G, Vassos E, Tatarelli R, Girardi P, Collier D, Frangou S (Dec 2009). "Effects of the CACNA1C risk allele for bipolar disorder on cerebral gray matter volume in healthy individuals". The American Journal of Psychiatry. 166 (12): 1413–4. doi:10.1176/appi.ajp.2009.09050680. PMID 19952088.
- Soldatov NM (May 1992). "Molecular diversity of L-type Ca2+ channel transcripts in human fibroblasts". Proceedings of the National Academy of Sciences of the United States of America. 89 (10): 4628–32. Bibcode:1992PNAS...89.4628S. doi:10.1073/pnas.89.10.4628. PMC 49136. PMID 1316612.
- Powers PA, Gregg RG, Hogan K (Sep 1992). "Linkage mapping of the human gene for the alpha 1 subunit of the cardiac DHP-sensitive Ca2+ channel (CACNL1A1) to chromosome 12p13.2-pter using a dinucleotide repeat". Genomics. 14 (1): 206–7. doi:10.1016/S0888-7543(05)80312-X. PMID 1330882.
- Sun W, McPherson JD, Hoang DQ, Wasmuth JJ, Evans GA, Montal M (Dec 1992). "Mapping of a human brain voltage-gated calcium channel to human chromosome 12p13-pter". Genomics. 14 (4): 1092–4. doi:10.1016/S0888-7543(05)80135-1. PMID 1335957.
- Powers PA, Gregg RG, Lalley PA, Liao M, Hogan K (Jul 1991). "Assignment of the human gene for the alpha 1 subunit of the cardiac DHP-sensitive Ca2+ channel (CCHL1A1) to chromosome 12p12-pter". Genomics. 10 (3): 835–9. doi:10.1016/0888-7543(91)90471-P. PMID 1653763.
- Perez-Reyes E, Wei XY, Castellano A, Birnbaumer L (Nov 1990). "Molecular diversity of L-type calcium channels. Evidence for alternative splicing of the transcripts of three non-allelic genes". The Journal of Biological Chemistry. 265 (33): 20430–6. doi:10.1016/S0021-9258(17)30522-7. PMID 2173707.
- Soldatov NM, Bouron A, Reuter H (May 1995). "Different voltage-dependent inhibition by dihydropyridines of human Ca2+ channel splice variants". The Journal of Biological Chemistry. 270 (18): 10540–3. doi:10.1074/jbc.270.18.10540. PMID 7737988.
- Soldatov NM (Jul 1994). "Genomic structure of human L-type Ca2+ channel". Genomics. 22 (1): 77–87. doi:10.1006/geno.1994.1347. PMID 7959794.
- Tang S, Mikala G, Bahinski A, Yatani A, Varadi G, Schwartz A (Jun 1993). "Molecular localization of ion selectivity sites within the pore of a human L-type cardiac calcium channel". The Journal of Biological Chemistry. 268 (18): 13026–9. doi:10.1016/S0021-9258(19)38613-2. PMID 8099908.
- Schultz D, Mikala G, Yatani A, Engle DB, Iles DE, Segers B, Sinke RJ, Weghuis DO, Klöckner U, Wakamori M (Jul 1993). "Cloning, chromosomal localization, and functional expression of the alpha 1 subunit of the L-type voltage-dependent calcium channel from normal human heart". Proceedings of the National Academy of Sciences of the United States of America. 90 (13): 6228–32. Bibcode:1993PNAS...90.6228S. doi:10.1073/pnas.90.13.6228. PMC 46901. PMID 8392192.
- Perets T, Blumenstein Y, Shistik E, Lotan I, Dascal N (Apr 1996). "A potential site of functional modulation by protein kinase A in the cardiac Ca2+ channel alpha 1C subunit". FEBS Letters. 384 (2): 189–92. doi:10.1016/0014-5793(96)00303-1. PMID 8612821. S2CID 40550657.
- Andersson B, Wentland MA, Ricafrente JY, Liu W, Gibbs RA (Apr 1996). "A "double adaptor" method for improved shotgun library construction". Analytical Biochemistry. 236 (1): 107–13. doi:10.1006/abio.1996.0138. PMID 8619474.
- Soldatov NM, Zühlke RD, Bouron A, Reuter H (Feb 1997). "Molecular structures involved in L-type calcium channel inactivation. Role of the carboxyl-terminal region encoded by exons 40-42 in alpha1C subunit in the kinetics and Ca2+ dependence of inactivation". The Journal of Biological Chemistry. 272 (6): 3560–6. doi:10.1074/jbc.272.6.3560. PMID 9013606.
- Klöckner U, Mikala G, Eisfeld J, Iles DE, Strobeck M, Mershon JL, Schwartz A, Varadi G (Mar 1997). "Properties of three COOH-terminal splice variants of a human cardiac L-type Ca2+-channel alpha1-subunit". The American Journal of Physiology. 272 (3 Pt 2): H1372–81. doi:10.1152/ajpheart.1997.272.3.H1372. PMID 9087614.
- Yu W, Andersson B, Worley KC, Muzny DM, Ding Y, Liu W, Ricafrente JY, Wentland MA, Lennon G, Gibbs RA (Apr 1997). "Large-scale concatenation cDNA sequencing". Genome Research. 7 (4): 353–8. doi:10.1101/gr.7.4.353. PMC 139146. PMID 9110174.
- Gao T, Yatani A, Dell'Acqua ML, Sako H, Green SA, Dascal N, Scott JD, Hosey MM (Jul 1997). "cAMP-dependent regulation of cardiac L-type Ca2+ channels requires membrane targeting of PKA and phosphorylation of channel subunits". Neuron. 19 (1): 185–96. doi:10.1016/S0896-6273(00)80358-X. PMID 9247274. S2CID 3253007.
- Zühlke RD, Bouron A, Soldatov NM, Reuter H (May 1998). "Ca2+ channel sensitivity towards the blocker isradipine is affected by alternative splicing of the human alpha1C subunit gene". FEBS Letters. 427 (2): 220–4. doi:10.1016/S0014-5793(98)00425-6. PMID 9607315. S2CID 32580111.
- Meyers MB, Puri TS, Chien AJ, Gao T, Hsu PH, Hosey MM, Fishman GI (Jul 1998). "Sorcin associates with the pore-forming subunit of voltage-dependent L-type Ca2+ channels". The Journal of Biological Chemistry. 273 (30): 18930–5. doi:10.1074/jbc.273.30.18930. PMID 9668070.
- Liu WS, Soldatov NM, Gustavsson I, Chowdhary BP (1999). "Fiber-FISH analysis of the 3'-terminal region of the human L-type Ca2+ channel alpha 1C subunit gene". Hereditas. 129 (2): 169–75. doi:10.1111/j.1601-5223.1998.00169.x. PMID 10022083.
External links
- GeneReviews/NIH/NCBI/UW entry on Brugada syndrome
- CACNA1C+protein,+human at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- GeneReviews/NIH/NCBI/UW entry on Timothy Syndrome
This article incorporates text from the United States National Library of Medicine, which is in the public domain.