Caecilian
Caecilians (New Latin for 'blind ones') (/sɪˈsɪliən/) are a group of limbless, vermiform (worm-shaped) or serpentine (snake-shaped) amphibians. They mostly live hidden in soil or in streambeds, and this cryptic lifestyle renders caecilians among the least familiar amphibians. Modern caecilians live in the tropics of South and Central America, Africa, and southern Asia. Caecilians feed on small subterranean creatures such as earthworms. The body is cylindrical and often darkly coloured, and the skull is bullet-shaped and strongly built. Caecilian heads have several unique adaptations, including fused cranial and jaw bones, a two-part system of jaw muscles, and a chemosensory tentacle in front of the eye. The skin is slimy and bears ringlike markings or grooves, which may contain tiny scales.
| Caecilian | |
|---|---|
.jpg.webp) | |
| Oscaecilia ochrocephala (Caeciliidae) | |
| Scientific classification | |
| Domain: | Eukaryota |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Class: | Amphibia |
| Subclass: | Lissamphibia |
| Order: | Gymnophiona Müller, 1832[1] |
| Subgroups | |
| Synonyms[1] | |
| |
Modern caecilians are grouped as a clade, Apoda /ˈæpədə/, one of three living amphibian groups alongside Anura (frogs) and Urodela (salamanders). Apoda is a crown group, encompassing modern, entirely limbless caecilians. There are more than 200 living species of caecilian distributed across 10 families. Many herpetologists name the caecilian total group as the order Gymnophiona /dʒɪmnəˈfaɪənə/, which includes Apoda as well as a few extinct stem-group caecilians (amphibians related to modern caecilians but evolving prior to the crown group).[2] Gymnophiona as a name derives from the Greek words γυμνος / gymnos (Ancient Greek for 'naked') and οφις / ophis (Ancient Greek for 'snake'), as the caecilians were originally thought to be related to snakes. The proper application of the names Apoda and Gymnophiona has been a matter of strong debate. Gymnophionomorpha is another name which has been applied to the caecilian total group.[3][4]
The study of caecilian evolution is complicated by their poor fossil record and specialized anatomy. Genetic evidence and some anatomical details (such as pedicellate teeth) support the idea that frogs, salamanders, and caecilians (collectively known as lissamphibians) are each others' closest relatives. Frogs and salamanders show many similarities to dissorophoids, a group of extinct amphibians in the order Temnospondyli. Caecilians are more controversial; many studies extend dissorophoid ancestry to caecilians. Some studies have instead argued that caecilians descend from extinct lepospondyl or stereospondyl amphibians, contradicting evidence for lissamphibian monophyly (common ancestry). Rare fossils of early gymnophionans such as Eocaecilia and Funcusvermis have helped to test the various conflicting hypotheses for the relationships between caecilians and other living and extinct amphibians.
Description

Caecilians anatomy is highly adapted for a burrowing lifestyle. They completely lack limbs, making the smaller species resemble worms, while the larger species like Caecilia thompsoni, with lengths up to 1.5 m (5 ft), resemble snakes. Their tails are short or absent, and their cloacae are near the ends of their bodies.[5][6][7]
Except for one lungless species, Atretochoana eiselti,[8] all caecilians have lungs, but also use their skin or mouths for oxygen absorption. Often, the left lung is much smaller than the right one, an adaptation to body shape that is also found in snakes.[9]
Their muscles are adapted to pushing their way through the ground, with the skeleton and deep muscles acting as a piston inside the skin and outer muscles. This allows the animal to anchor its hind end in position, and force the head forwards, and then pull the rest of the body up to reach it in waves. In water or very loose mud, caecilians instead swim in an eel-like fashion.[10] Caecilians in the family Typhlonectidae are aquatic, and the largest of their kind. The representatives of this family have a fleshy fin running along the rear section of their bodies, which enhances propulsion in water.[11]
Skull and senses
Caecilians have small or absent eyes, with only a single known class of photoreceptors, and their vision is limited to dark-light perception.[12][13] Unlike other modern amphibians (frogs and salamanders) the skull is compact and solid, with few large openings between plate-like cranial bones. The snout is pointed and bullet-shaped, used to force their way through soil or mud. In most species the mouth is recessed under the head, so that the snout overhangs the mouth.[7]
The bones in the skull are reduced in number compared to prehistoric amphibian species. Many bones of the skull are fused together: the maxilla and palatine bones have fused into a maxillopalatine in all living caecilians, and the nasal and premaxilla bones fuse into a nasopremaxilla in some families. Some families can be differentiated by the presence of absence of certain skull bones, such as the septomaxillae, prefrontals, an/or a postfrontal-like bone surrounding the orbit (eye socket). The braincase is encased in a fully integrated compound bone called the os basale, which takes up most of the rear and lower parts of the skull. In skulls viewed from above, a mesethmoid bone may be visible in some species, wedging into the midline of the skull roof.[14][15][16]

All caecilians have a pair of unique sensory structures, known as tentacles, located on either side of the head between the eyes and nostrils. These are probably used for a second olfactory capability, in addition to the normal sense of smell based in the nose.[10]
The ringed caecilian (Siphonops annulatus) has dental glands that may be homologous to the venom glands of some snakes and lizards. The function of these glands is unknown.[17]
The middle ear consists of only the stapes bone and the oval window, which transfer vibrations into the inner ear through a reentrant fluid circuit as seen in some reptiles. Species within the family Scolecomorphidae lack both a stapes and an oval window, making them the only known amphibians missing all the components of a middle ear apparatus.[18]
The lower jaw is specialized in caecilians. Gymnophionans, including extinct species, have only two components of the jaw: the pseudodentary (at the front, bearing teeth) and pseudoangular (at the back, bearing the jaw joint and muscle attachments). These two components are what remains following fusion between a larger set of bones. An additional inset tooth row with up to 20 teeth lies parallel to the main marginal tooth row of the jaw.[15]
All but the most primitive caecilians have two sets of muscles for closing the jaw, compared with the single pair found in other amphibians. One set of muscles, the adductors, insert into the upper edge of the pseudoangular in front of the jaw joint. Adductor muscles are commonplace in vertebrates, and close the jaw by pulling upwards and forwards. A more unique set of muscles, the abductors, insert into the rear edge of the pseudoangular below and behind the jaw joint. They close the jaw by pulling backwards and downwards. Jaw muscles are more highly developed in the most efficient burrowers among the caecilians, and appear to help keep the skull and jaw rigid.[10][19]
Skin

Their skin is smooth and usually dark, but some species have colourful skins. Inside the skin are calcite scales. Because of these scales, the caecilians were once thought to be related to the fossil Stegocephalia, but they are now believed to be a secondary development, and the two groups are most likely unrelated.[7] Scales are absent in the families Scolecomorphidae and Typhlonectidae, except the species Typhlonectes compressicauda where minute scales have been found in the hinder region of the body.[20] The skin also has numerous ring-shaped folds, or annuli, that partially encircle the body, giving them a segmented appearance. Like some other living amphibians, the skin contains glands that secrete a toxin to deter predators.[10] The skin secretions of Siphonops paulensis have been shown to have hemolytic properties.[21]
Distribution

Caecilians are native to wet, tropical regions of Southeast Asia, India, Bangladesh, Nepal[22] and Sri Lanka, parts of East and West Africa, the Seychelles Islands in the Indian Ocean, Central America, and in northern and eastern South America. In Africa, caecilians are found from Guinea-Bissau (Geotrypetes) to southern Malawi (Scolecomorphus), with an unconfirmed record from eastern Zimbabwe. They have not been recorded from the extensive areas of tropical forest in central Africa. In South America, they extend through subtropical eastern Brazil well into temperate northern Argentina. They can be seen as far south as Buenos Aires, when they are carried by the flood waters of the Paraná River coming from farther north. Their American range extends north to southern Mexico. The northernmost distribution is of the species Ichthyophis sikkimensis of northern India. Ichthyophis is also found in South China and Northern Vietnam. In Southeast Asia, they are found as far east as Java, Borneo, and the southern Philippines, but they have not crossed Wallace's line and are not present in Australia or nearby islands. There are no known caecilians in Madagascar, but their presence in the Seychelles and India has led to speculation on the presence of undiscovered extinct or extant caecilians there.[23]
In 2021, a live specimen of Typhlonectes natans, a caecilian native to Colombia and Venezuela, was collected from a drainage canal in South Florida. It was the only caecilian ever reported in the wild in the United States, and is considered to be an introduction, perhaps from the wildlife trade. Whether a breeding population has been established in the area is unknown.[24][25]
Taxonomy
The name caecilian derives from the Latin word caecus, meaning "blind", referring to the small or sometimes nonexistent eyes. The name dates back to the taxonomic name of the first species described by Carl Linnaeus, which he named Caecilia tentaculata.[7]
There has historically been disagreement over the use of the two primary scientific names for caecilians, Apoda and Gymnophiona. Some specialists prefer to use the name Gymnophiona to refer to the "crown group", that is, the group containing all modern caecilians and extinct members of these modern lineages. They sometimes use the name Apoda to refer to the total group, that is, all caecilians and caecilian-like amphibians that are more closely related to modern groups than to frogs or salamanders. However, many scientists have advocated for the reverse arrangement, where Apoda is used as the name for modern caecilian groups. Some have argued that this use makes more sense, because the name "Apoda" means "without feet", and this is a feature associated mainly with modern species (some stem-group caecilian-like amphibians, such as Eocaecilia, had legs).[26] The name "Gymniophonomorpha" has been proposed to avert this disagreement, acting as the total group with Gymnophiona as the crown group.[3]
A classification of caecilians by Wilkinson et al. (2011) divided the caecilians into 9 families containing nearly 200 species.[15] In 2012, a tenth caecilian family was newly described, Chikilidae.[27][28] This classification is based on a thorough definition of monophyly based on morphological and molecular evidence,[29][30][31][32] and it solves the longstanding problems of paraphyly of the Caeciliidae in previous classifications without an exclusive reliance upon synonymy.[15][33] There are 219 species of caecilian in 33 genera and 10 families.
| Family | Image | Taxon author | Genera | Species | Common name | Geographic range |
|---|---|---|---|---|---|---|
| Caeciliidae |  Caecilia subnigricans |
Rafinesque, 1814 | 2 | 47 | Common caecilians | Central and South America (Bolivia north to Costa Rica). |
| Chikilidae | Kamei et al., 2012 | 1 | 4 | Northeast Indian caecilians | Northeast India and Bangladesh, with possible occurrences in Myanmar. | |
| Dermophiidae |  Geotrypetes seraphini |
Taylor, 1969 | 4 | 15 | Neotropical caecilians | Equatorial Africa (West Africa, Tanzania, Kenya), Central and South America (Colombia north to Mexico). |
| Grandisoniidae (formerly Indotyphlidae) |  Grandisonia sechellensis |
Lescure, Renous & Gasc, 1986 | 7 | 24 | Indo-African caecilians | Equatorial Africa (Cameroon, Ethiopia), the Seychelles, western India (Western Ghats). |
| Herpelidae |  Boulengerula taitana |
Laurent, 1984 | 2 | 10 | African caecilians | Equatorial Africa (Nigeria south to the Democratic Republic of the Congo, Kenya south to Malawi, possible occurrences in Angola and Zambia). |
| Ichthyophiidae |  Ichthyophis kodaguensis |
Taylor, 1969 | 2 | 57 | Asian tailed caecilians | South and Southeast Asia (western India north to Nepal, east to the Philippines, southern China and Indonesia). |
| Rhinatrematidae |  Epicrionops sp. |
Nussbaum, 1977 | 3 | 14 | American tailed caecilians | Northern South America (northernmost Brazil west to Venezuela, Colombia, Ecuador, and Peru). |
| Scolecomorphidae | .jpg.webp) Scolecomorphus kirkii |
Taylor, 1969 | 2 | 6 | Buried-eyed caecilians | Equatorial Africa (Cameroon, Tanzania, Malawi, Mozambique). |
| Siphonopidae |  Microcaecilia dermatophaga |
Bonaparte, 1850 | 5 | 28 | South American caecilians | South America (Colombia south to northern Argentina, Paraguay, and southernmost Brazil). |
| Typhlonectidae | Typhlonectes natans |
Taylor, 1968 | 5 | 14 | Aquatic caecilians | South America (Colombia and Venezuela south to northern Argentina and Uruguay). |
The most recent phylogeny of caecilians is based on molecular mitogenomic evidence examined by San Mauro et al. (2014), and modified to include some more recently described genera such as Amazops.[34][35][36]
| Gymnophiona |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Evolution

Little is known of the evolutionary history of the caecilians, which have left a very sparse fossil record. The first fossil, a vertebra dated to the Paleocene, was not discovered until 1972.[37] Other vertebrae, which have characteristic features unique to modern species, were later found in Paleocene and Late Cretaceous (Cenomanian) sediments.[2]
Phylogenetic evidence suggests that the ancestors of caecilians and batrachians (including frogs and salamanders) diverged from one another during the Carboniferous. This leaves a gap of more than 70 million years between the presumed origins of caecilians and the earliest definitive fossils of stem-caecilians.[38][4]
Prior to 2023, the earliest fossil attributed to a stem-caecilian (an amphibian closer to caecilians than to frogs or salamanders but not a member of the extant caecilian lineage) comes from the Jurassic period. This primitive genus, Eocaecilia, had small limbs and well-developed eyes.[39] In their 2008 description of the Early Permian amphibian Gerobatrachus,[40] Anderson and co-authors suggested that caecilians arose from the Lepospondyl group of ancestral tetrapods, and may be more closely related to amniotes than to frogs and salamanders, which arose from Temnospondyl ancestors. Numerous groups of lepospondyls evolved reduced limbs, elongated bodies, and burrowing behaviors, and morphological studies on Permian and Carboniferous lepospondyls have placed the early caecilian (Eocaecilia) among these groups.[41] Divergent origins of caecilians and other extant amphibians may help explain the slight discrepancy between fossil dates for the origins of modern Amphibia, which suggest Permian origins, and the earlier dates, in the Carboniferous, predicted by some molecular clock studies of DNA sequences. Most morphological and molecular studies of extant amphibians, however, support monophyly for caecilians, frogs, and salamanders, and the most recent molecular study based on multi-locus data suggest a Late Carboniferous–Early Permian origin of extant amphibians.[42]
Chinlestegophis, a stereospondyl temnospondyl from the Late Triassic Chinle Formation of Colorado, was proposed to be a stem-caecilian in a 2017 paper by Pardo and co-authors. If confirmed, this would bolster the proposed pre-Triassic origin of Lissamphibia suggested by molecular clocks. It would fill a gap in the fossil record of early caecilians and suggest that stereospondyls as a whole qualify as stem-group caecilians.[38] However, affinities between Chinlestegophis and gymnophionans have been disputed along several lines of evidence. A 2020 study questioned the choice of characters supporting the relationship,[3] and a 2019 reanalysis of the original data matrix found that other equally parsimonious positions were supported for the placement of Chinlestegophis and gymnophionans among tetrapods.[43]
A 2023 paper by Kligman and co-authors described Funcusvermis, another amphibian from the Chinle Formation of Arizona. Funcusvermis was strongly supported as a stem group caecilian based on traits of its numerous skull and jaw fragments, the largest sample of caecilian fossils known. The paper discussed the various hypotheses for caecilian origins: the polyphyly hypothesis (caecilians as lepospondyls, and other lissamphibians as temnospondyls), the lepospondyl hypothesis (lissamphibians as lepospondyls), and the newer hypothesis supported by Chinlestegophis, where caecilians and other lissamphibians had separate origins within temnospondyls. Nevertheless, all of these ideas were refuted, and the most strongly supported hypothesis combined lissamphibians into a monophyletic group of dissorophoid temnospondyls closely related to Gerobatrachus.[4]
Behavior
Reproduction
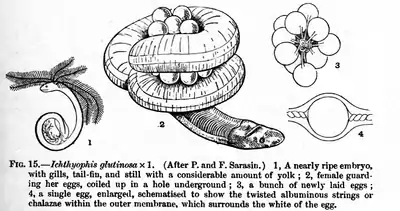
Caecilians are the only order of amphibians to use internal insemination exclusively (although most salamanders have internal fertilization and the tailed frog in the US uses a tail-like appendage for internal insemination in its fast-flowing water environment).[10] The male caecilians have a long tube-like intromittent organ, the phallodeum,[44] which is inserted into the cloaca of the female for two to three hours. About 25% of the species are oviparous (egg-laying); the eggs are laid in terrestrial nests rather than in water and are guarded by the female. For some species, the young caecilians are already metamorphosed when they hatch; others hatch as larvae. The larvae are not fully aquatic, but spend the daytime in the soil near the water.[10][45]
About 75% of caecilians are viviparous, meaning they give birth to already-developed offspring. The foetus is fed inside the female with cells lining the oviduct, which they eat with special scraping teeth. Some larvae, such as those of Typhlonectes, are born with enormous external gills which are shed almost immediately.

The egg-laying herpelid species Boulengerula taitana feeds its young by developing an outer layer of skin, high in fat and other nutrients, which the young peel off with modified teeth. This allows them to grow by up to 10 times their own weight in a week. The skin is consumed every three days, the time it takes for a new layer to grow, and the young have only been observed to eat it at night. It was formerly thought that the juveniles subsisted only on a liquid secretion from their mothers.[46][47][48] This form of parental care, known as maternal dermatophagy, has also been reported in two species in the family Siphonopidae: Siphonops annulatus and Microcaecilia dermatophaga. Siphonopids and herpelids are not closely related to each other, having diverged in the Cretaceous Period. The presence of maternal dermatophagy in both families suggest that it may be more widespread among caecilians than previously considered.[49][50]
Herpele squalostoma caecilians vertically transmit the mother's microbiome to their offspring through maternal dermatophagy. In comparison to other amphibians, the extended parenting of caecilians can provide beneficial bacteria and fungi, but this transmission risks the spread of diseases like chytridiomycosis.[51][52]
Diet
While caecilians are generally carnivorous, their diet differs between taxa. The stomach contents of wild caecilians include earthworms, termites, lizards, moth larvae, and shrimp. Some species of caecilians will opportunistically consume newborn rodents, salmon eggs, and veal in laboratory conditions.[53]
Cultural significance
As caecilians are a reclusive group, they are only featured in a few human myths, and are generally considered repulsive in traditional customs.
In the folklore of certain regions of India, caecilians are feared and reviled, based on the belief that they are fatally venomous. Caecilians in the Eastern Himalayas are colloquially known as "back ache snakes",[54] while in the Western Ghats, Ichthyophis tricolor is considered to be more toxic than a king cobra.[55][56] Despite deep cultural respect for the cobra and other dangerous animals, the caecilian is killed on sight by salt and kerosene.[55] These myths have complicated conservation initiatives for Indian caecilians.[55][54][56]
Crotaphatrema lamottei, a rare species native to Mount Oku in Cameroon, is classified as a Kefa-ntie (burrowing creature) by the Oku. Kefa-ntie, a term also encompassing native moles and blind snakes, are considered poisonous, causing painful sores if encountered, contacted, or killed. According to Oku tradition, the ceremony to cleanse the affliction involves a potion composed of ground herbs, palm oil, snail shells, and chicken blood applied to and licked off of the left thumb.[57]
South American caecilians have a variable relationship to local cultures.[56] The minhocão, a legendary worm-like beast in Brazilian folklore, may be inspired by caecilians. Colombian folklore states that the aquatic caecilian, Typhlonectes natans, can be manifested from a lock of hair sealed in a sunken bottle. In southern Mexico and Central America, Dermophis mexicanus is colloquially known as the "tapalcua", a name referencing the belief that it emerges to embed itself in the rear end of any unsuspecting person who chooses to relieve themself over its home. This may be inspired by their tendency to nest in refuse heaps.[56]
See also
References
- Frost, Darrel R. (2019). "Gymnophiona Müller, 1832". Amphibian Species of the World: an Online Reference. Version 6.0. American Museum of Natural History. Retrieved 11 September 2019.
- Evans, Susan E.; Sigogneau-Russell, Denise (2001). "A stem-group caecilian (Lissamphibia: Gymnophiona) from the Lower Cretaceous of North Africa". Palaeontology. 44 (2): 259–273. doi:10.1111/1475-4983.00179.
- Santos, Rodolfo Otávio; Laurin, Michel; Zaher, Hussam (2020). "A review of the fossil record of caecilians (Lissamphibia; Gymnophionomorpha) with comments on its use to calibrate molecular timetrees". Biological Journal of the Linnean Society. 131 (4): 737–755. doi:10.1093/biolinnean/blaa148.
- Kligman, Ben T.; Gee, Bryan M.; Marsh, Adam D.; Nesbitt, Sterling J.; Smith, Matthew E.; Parker, William G.; Stocker, Michelle R. (25 January 2023). "Triassic stem caecilian supports dissorophoid origin of living amphibians". Nature. 614 (7946): 102–107. doi:10.1038/s41586-022-05646-5. ISSN 1476-4687. PMC 9892002. PMID 36697827.
- Goin, C. J.; Goin, O.B.; Zug, G.W. (1978). "Order Gymnophiona". Introduction to Herpetology (3rd ed.). San Francisco: W.H. Freeman and Company. p. 201. ISBN 978-0-7167-0020-3.
- Himstedt, Werner (1996). Die Blindwühlen (in German). Magdeburg: Westarp Wissenschaften. ISBN 978-3-89432-434-6.
- Chisholm, Hugh, ed. (1911). . Encyclopædia Britannica (11th ed.). Cambridge University Press.
- "Atretochoana eiselti". Natural History Museum. Retrieved 22 February 2012.
- Mader D (June 1995). "Reptilian Anatomy". Reptiles. 3 (2): 84–93.
- Nussbaum, Ronald A. (1998). Cogger, H.G.; Zweifel, R.G. (eds.). Encyclopedia of Reptiles and Amphibians. San Diego: Academic Press. pp. 52–59. ISBN 978-0-12-178560-4.
- Piper, Ross (2007). Extraordinary Animals: An Encyclopedia of Curious and Unusual Animals. Greenwood Press.
- The Evolution of Amphibian Photoreception - Frontiers
- Mohun, S. M.; Davies, W. L.; Bowmaker, J. K.; Pisani, D.; Himstedt, W.; Gower, D. J.; Hunt, D. M.; Wilkinson, M. (2010). "Identification and characterization of visual pigments in caecilians (Amphibia: Gymnophiona), an order of limbless vertebrates with rudimentary eyes". The Journal of Experimental Biology. 213 (20): 3586–3592. doi:10.1242/jeb.045914. PMID 20889838.
- Taylor, Edward Harrison (1969). "Skulls of Gymnophiona and their significance in the taxonomy of the group". The University of Kansas Science Bulletin. 48 (15): 585–687.
- Wilkinson, M.; San Mauro, D.; Sherratt, E.; Gower, D.J. (2011). "A nine-family classification of caecilians (Amphibia: Gymnophiona)" (PDF). Zootaxa. 2874: 41–64. doi:10.11646/zootaxa.2874.1.3. S2CID 86301415.
- Marshall, Ashleigh F; Bardua, Carla; Gower, David J; Wilkinson, Mark; Sherratt, Emma; Goswami, Anjali (27 March 2019). "High-density three-dimensional morphometric analyses support conserved static (intraspecific) modularity in caecilian (Amphibia: Gymnophiona) crania". Biological Journal of the Linnean Society. 126 (4): 721–742. doi:10.1093/biolinnean/blz001. hdl:2440/123277. ISSN 0024-4066.
- Mailho-Fontana, Pedro Luiz; Antoniazzi, Marta Maria; Alexandre, Cesar; Pimenta, Daniel Carvalho; Sciani, Juliana Mozer; Brodie, Edmund D.; Jared, Carlos (July 2020). "Morphological Evidence for an Oral Venom System in Caecilian Amphibians". iScience. 23 (7): 101234. Bibcode:2020iSci...23j1234M. doi:10.1016/j.isci.2020.101234. ISSN 2589-0042. PMC 7385905. PMID 32621800.
- Hearing and Sound Communication in Amphibians
- Kleinteich, Thomas; Haas, Alexander; Summers, Adam P (15 May 2008). "Caecilian jaw-closing mechanics: integrating two muscle systems". Journal of the Royal Society Interface. 5 (29): 1491–1504. doi:10.1098/rsif.2008.0155. PMC 2607354. PMID 18482905.
- Arun, Damodaran; Sandhya, S.; Akbarsha, Mohammad Abdulkader; Oommen, Oommen V.; Divya, Lekha (2020). "An insight into the skin glands, dermal scales and secretions of the caecilian amphibian Ichthyophis beddomei". Saudi Journal of Biological Sciences. 27 (10): 2683–2690. doi:10.1016/j.sjbs.2020.06.009. PMC 7499274. PMID 32994727.
- Elisabeth N. Ferroni Schwartz; Carlos A. Schwartz; Antonio Sebben (1998). "Occurrence of hemolytic activity in the skin secretion of the caecilian Siphonops paulensis". Natural Toxins. 6 (5): 179–182. doi:10.1002/(SICI)1522-7189(199809/10)6:5<179::AID-NT20>3.0.CO;2-M. PMID 10398514.
- Rathor, Hariharsingh (5 May 2016). "Kutnjema Mareka Jiv Sarpa Hoina". Katipur. Kantipur News. Archived from the original on 22 February 2017. Retrieved 5 May 2016.
- James D. Gardner, Jean-Claude Rage, The fossil record of lissamphibians from Africa, Madagascar, and the Arabian Plate, Palaeobiodiversity and Palaeoenvironments 96(1):1-52 · March 2016
- "Worm-Like, Limbless Amphibian Known As Caecilians Spotted in Florida For The First Time". Conservation.Reefcause.com. 3 August 2021. Retrieved 4 August 2021.
- Sheehy, Coleman; Blackburn, David; Kouete, Marcel; Gestring, Kelly; Laurie, Kristin; Prechtel, Austin; Suarez, Eric; Talley, Brooke (15 July 2021). "First record of a caecilian (order Gymnophiona, Typhlonectes natans) in Florida and in the United States". Reptiles & Amphibians. 28 (2): 355–357. doi:10.17161/randa.v28i2.15629. ISSN 2332-4961.
- Marjanovic, D.; Laurin, M. (2007). "Fossils, molecules, divergence times, and the origin of lissamphibians". Syst. Biol. 56 (3): 369–388. doi:10.1080/10635150701397635. PMID 17520502.
- Kamei, R.G.; San Mauro, D.; Gower, D. J.; Van Bocxlaer, I.; Sherratt, E.; Thomas, A.; Babu, S.; Bossuyt, F.; Wilkinson, M.; Biju, S. D. (2012). "Discovery of a new family of amphibians from Northeast India with ancient links to Africa". Proc. R. Soc. B. 279 (1737): 2396–401. doi:10.1098/rspb.2012.0150. PMC 3350690. PMID 22357266.
- "New amphibian family found in India". CBC News. Associated Press. 21 February 2012.
- San Mauro, D.; Gower, D. J.; Oommen, O. V.; Wilkinson, M.; Zardoya, R. (2004). "Phylogeny of caecilian amphibians (Gymnophiona) based on complete mitochondrial genomes and nuclear RAG1". Molecular Phylogenetics and Evolution. 33 (2): 413–427. doi:10.1016/j.ympev.2004.05.014. PMID 15336675.
- San Mauro, D.; Gower, D. J.; Massingham, T.; Wilkinson, M.; Zardoya, R.; Cotton, J. A. (2009). "Experimental design in caecilian systematics: phylogenetic information of mitochondrial genomes and nuclear rag1". Systematic Biology. 58 (4): 425–438. CiteSeerX 10.1.1.577.2856. doi:10.1093/sysbio/syp043. PMID 20525595.
- Zhang, P.; Wake, M. H. (2009). "A mitogenomic perspective on the phylogeny and biogeography of living caecilians (Amphibia: Gymnophiona)". Molecular Phylogenetics and Evolution. 53 (2): 479–491. doi:10.1016/j.ympev.2009.06.018. PMID 19577653.
- San Mauro, D.; Gower, D. J.; Cotton, J. A.; Zardoya, R.; Wilkinson, M.; Massingham, T. (2012). "Experimental design in phylogenetics: testing predictions from expected information". Systematic Biology. 61 (4): 661–674. doi:10.1093/sysbio/sys028. PMID 22328568.
- Frost, Darrel R.; Grant, Taran; Faivovich, Julián; Bain, Raoul H.; Haas, Alexander; Haddad, Célio F.B.; De Sá, Rafael O.; Channing, Alan; Wilkinson, Mark; Donnellan, Stephen C.; Raxworthy, Christopher J.; Campbell, Jonathan A.; Blotto, Boris L.; Moler, Paul; Drewes, Robert C.; Nussbaum, Ronald A.; Lynch, John D.; Green, David M.; Wheeler, Ward C. (2006). "The Amphibian Tree of Life". Bulletin of the American Museum of Natural History. 297: 1–370, appendices. doi:10.1206/0003-0090(2006)297[0001:TATOL]2.0.CO;2. ISSN 0003-0090. S2CID 86140137.
- Pyron, R.A.; Wiens, J.J. (2011). "A large-scale phylogeny of Amphibia including over 2800 species, and a revised classification of extant frogs, salamanders, and caecilians". Molecular Phylogenetics and Evolution. 61 (2): 543–583. doi:10.1016/j.ympev.2011.06.012. PMID 21723399.
- San Mauro, D.; Gower, D. J.; Müller, H.; Loader, S. P.; Zardoya, R.; Nussbaum, R. A.; Wilkinson, M. (2014). "Life-history evolution and mitogenomic phylogeny of caecilian amphibians". Molecular Phylogenetics and Evolution. 73: 177–89. doi:10.1016/j.ympev.2014.01.009. hdl:10261/123960. PMID 24480323.
- "AmphibiaWeb - Amazops amazops". amphibiaweb.org. Retrieved 24 January 2021.
- Estes, Richard; Wake, Marvalee H. (22 September 1972). "The First Fossil Record of Caecilian Amphibians". Nature. 239 (5369): 228–231. Bibcode:1972Natur.239..228E. doi:10.1038/239228b0. S2CID 4260251.
- Pardo, Jason D.; Small, Bryan J.; Huttenlocker, Adam K. (3 July 2017). "Stem caecilian from the Triassic of Colorado sheds light on the origins of Lissamphibia". Proceedings of the National Academy of Sciences. 114 (27). doi:10.1073/pnas.1706752114. ISSN 0027-8424. PMC 5502650. PMID 28630337.
- Jenkins, Parish A.; Walsh, Denis M. (16 September 1993). "An Early Jurassic caecilian with limbs". Nature. 365 (6443): 246–250. Bibcode:1993Natur.365..246J. doi:10.1038/365246a0. S2CID 4342438.
- Anderson, Jason S.; Reisz, Robert R.; Scott, Diane; Fröbisch, Nadia B.; Sumida, Stuart S. (2008). "A stem batrachian from the Early Permian of Texas and the origin of frogs and salamanders". Nature. 453 (7194): 515–8. Bibcode:2008Natur.453..515A. doi:10.1038/nature06865. PMID 18497824. S2CID 205212809.
- Huttenlocker, A. K.; Pardo, J. D.; Small, B. J.; Anderson, J. S. (2013). "Cranial morphology of recumbirostrans (Lepospondyli) from the Permian of Kansas and Nebraska, and early morphological evolution inferred by micro-computed tomography". Journal of Vertebrate Paleontology. 33 (3): 540–552. doi:10.1080/02724634.2013.728998. S2CID 129144343.
- San Mauro, D. (2010). "A multilocus timescale for the origin of extant amphibians". Molecular Phylogenetics and Evolution. 56 (2): 554–561. doi:10.1016/j.ympev.2010.04.019. PMID 20399871.
- Marjanović, David; Laurin, Michel (2019). "Phylogeny of Paleozoic limbed vertebrates reassessed through revision and expansion of the largest published relevant data matrix". PeerJ. 6 (e5565): e5565. doi:10.7717/peerj.5565. PMC 6322490. PMID 30631641.
- Jamieson, Barrie G. M. (2006). Reproductive Biology and Phylogeny of Gymnophiona: Caecilians. CRC Press. ISBN 978-1-4822-8014-2.
- Caecilians - Cell Press
- Kupfer, Alex; Muller, Hendrik; Antoniazzi, Marta M.; Jared, Carlos; Greven, Hartmut; Nussbaum, Ronald A.; Wilkinson, Mark (2006). "Parental investment by skin feeding in a caecilian amphibian" (PDF). Nature. 440 (7086): 926–929. Bibcode:2006Natur.440..926K. doi:10.1038/nature04403. hdl:2027.42/62957. PMID 16612382. S2CID 4327433.
- Vince, Gaia (12 April 2006). "'Yummy mummy' worms feed their skin to offspring". New Scientist.
- Kupfer, Alexander; Wilkinson, Mark; Gower, David J.; Müller, Hendrik; Jehle, Robert (2008). "Care and parentage in a skin-feeding caecilian amphibian". Journal of Experimental Zoology Part A: Ecological Genetics and Physiology. 309A (8): 460–467. doi:10.1002/jez.475. PMID 18618577.
- Wilkinson, Mark; Kupfer, Alexander; Marques-Porto, Rafael; Jeffkins, Hilary; Antoniazzi, Marta M; Jared, Carlos (11 June 2008). "One hundred million years of skin feeding? Extended parental care in a Neotropical caecilian (Amphibia: Gymnophiona)". Biology Letters. 4 (4): 358–361. doi:10.1098/rsbl.2008.0217. PMC 2610157. PMID 18547909.
- Wilkinson, Mark; Sherratt, Emma; Starace, Fausto; Gower, David J. (6 March 2013). "A New Species of Skin-Feeding Caecilian and the First Report of Reproductive Mode in Microcaecilia (Amphibia: Gymnophiona: Siphonopidae)". PLOS ONE. 8 (3): e57756. Bibcode:2013PLoSO...857756W. doi:10.1371/journal.pone.0057756. ISSN 1932-6203. PMC 3590283. PMID 23483926.
- Quaglia, Sofia (18 October 2023). "These Amphibians Have a Taste for Their Mom's Skin". The New York Times. Retrieved 22 October 2023.
- Kouete, Marcel T.; Bletz, Molly C.; LaBumbard, Brandon C.; Woodhams, Douglas C.; Blackburn, David C. (15 May 2023). "Parental Care Contributes to Vertical Transmission of Microbes in a Skin-Feeding and Direct-Developing Caecilian". Animal Microbiome. BioMed Central. 5 (28). doi:10.1186/s42523-023-00243-x.
- Schwenk, Kurt (2000). Feeding: Form, Function, and Evolution in Tetrapod Vertebrates. Elsevier. pp. 149–167. ISBN 9780080531632.
- Sathyabhamu, Das Biju, Rachunliu G Kamei, David Gower, & Mark Wilkinson (2009) "Conservation of Caecilians in the Eastern Himalayas Region" Critical Ecosystem Partnership Fund Project Report. pp. 1–22.
- K. Ramachandran & Oommen V. Oommen (August 2008) "Deep-rooted myths and their impact on the population of gymnophionan amphibians among the inhabited areas of Kerala, India" FrogLog v. 88. pp 3–5.
- Crump, Marty (2015). Eye of Newt and Toe of Frog, Adder's Fork and Lizard's Leg. University of Chicago Press. doi:10.7208/chicago/9780226116143.001.0001. ISBN 978-0-226-11600-6.
- Doherty-Bone, Thomas M.; Ndifon, R.K.; Gower, David J. (2011). "Traditional indigenous perspectives on soil-dwelling vertebrates in Oku, Cameroon, with special reference to the caecilian Crotaphatrema lamottei". Herpetological Bulletin. 116: 19–24.
External links
 Media related to Gymnophiona at Wikimedia Commons
Media related to Gymnophiona at Wikimedia Commons Data related to Gymnophiona at Wikispecies
Data related to Gymnophiona at Wikispecies