Carbetocin
Carbetocin, sold under the brand names Pabal among others, is a medication used to prevent excessive bleeding after childbirth, particularly following Cesarean section.[2] It appears to work as well as oxytocin.[3] Due to it being less economical than other options, use is not recommended by NHS Scotland.[2] It is given by injection into a vein or muscle.[3]
 | |
| Clinical data | |
|---|---|
| Trade names | Duratocin, Pabal, Lonactene, others |
| Other names | (2-O-Methyltyrosine)deamino-1-carbaoxytocin; Deamino-2-O-methyltyrosine-1-carbaoxytocin; 1-Butanoic acid-2-(O-methy-L-tyrosine)-1-carbaoxytocin; 1-butyric acid-2-[3-(4-methoxyphenyl)-L-alanine]oxytocin |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| Routes of administration | Intravenous, intramuscular |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 80% (IM) |
| Elimination half-life | 85–100 minutes[1] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.048.450 |
| Chemical and physical data | |
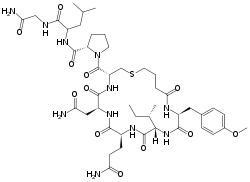
| Formula | C45H69N11O12S |
| Molar mass | 988.17 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Side effects differ little from that of no treatment or placebo.[3] Use is not recommended in people with epilepsy or eclampsia.[2] Carbetocin is a manufactured long acting form of oxytocin.[4] It works by activating the oxytocin receptor which causes the uterus to contract.[5][4]
Carbetocin was first described in 1974.[6] It was approved for medical use in Canada and the United Kingdom in 1997.[3] It is on the World Health Organization's List of Essential Medicines.[7] It is not available in the United States or Japan.[8][4]
Medical uses
Carbetocin has been approved for use immediately following an elective Cesarean section when a local or spinal anesthesia has been used.[9] Since the uterus cannot contract on its own following incision during a Cesarean section, exogenous administration of oxytocin or an analog is necessary to restore uterine tone and prevent hemorrhage.[9][10]
Safety of carbetocin following vaginal births and emergency Cesarean sections has not been established, though studies have suggested efficacy following vaginal births to that following Cesarean sections. Some studies have shown that a 10-70 ug dose following vaginal delivery caused contractions and no adverse side effects.[11] Carbetocin has also been shown to increase uterine involution (the return of the uterus to its contracted state after the birth of the baby) in humans, horses and cows.[12][13]
Comparison with other medication
In 2018, heat-stable carbetocin, a formulation that does not require strict refrigeration, was found to be as good as oxytocin for reduction of postpartum hemorrhage after vaginal delivery.[14] It is hoped that this will make oxytocic hemorrhage control more widely available and less expensive,[14] which will be particularly useful in regions of developing countries where the cold chain (in drug transport and storage) is unreliable because of power outages or equipment problems.[15][16]
Due to carbetocin's considerably longer half-life, its effects are longer lasting than other oxytocin homologs such as oxytocin or barusiban.[17] A single carbetocin dose compared to a placebo or an eight-hour intravenous drip of oxytocin in a randomized blind study, necessitated less additional oxytocin therapy following a Cesarean section. Oxytocin receptor antagonists, such as barusiban or atosiban have the opposite effect of depressing oxytocin receptor activity and can be used to stop premature labor and uterine contractions.[17]
Adverse effects
Ten to forty percent of people will experience nausea, vomiting, abdominal pain, itching skin, increased body temperature, trembling and weakness. One to five percent of peoples may experience back and chest pain, dizziness, anemia, chills and sweating, metallic taste, tachycardia and respiratory distress.[18][19][20]
Contraindications for the use of carbetocin include inappropriate timing during labor and delivery (such as before parturition or to induce labor) or allergic reactions to carbetocin or other oxytocin homologues.[18] Additionally, carbetocin should not be used if a person has high blood pressure or cardiovascular problems. Overdosage or repeated use of carbetocin, particularly if used during pregnancy, could cause hyper-excitation of the oxytocin receptors resulting in excessive and prolonged stimulation of uterine contractions, increasing risk of uterine rupture, placental abruption, fetal respiratory distress and postpartum hemorrhage.[18]
Interactions
Due to oxytocin's close sequence homology with vasopressin, oxytocin analogs often bind with much lower affinity to vasopressin receptors V1, in the uterine lining, and V2, in the kidneys[20] and may consequently interact with or disrupt the vasopressin circuitry and feedback loops. Carbetocin may work synergistically with drugs such as dinoprostone and misoprostol that ripen the cervix. Concurrent use of these drugs can be risky, particularly during pregnancy and prenatal care, possibly causing premature labor or abortion.
Pharmacology
Mechanism of action
Carbetocin works as an oxytocic, antihemorrhagic and uterotonic drug in the peripheral nervous system. The most common causes of postpartum hemorrhage are lack of tone in the uterus from overstretching or the use of an anesthetic.[21]
Carbetocin functions as an agonist at peripheral oxytocin receptors, particularly in the myometrium, with lesser affinity for myoepithelial cells. Oxytocin receptors are G protein-coupled[22] and their mechanism of action involves second messengers and the production of inositol phosphates.[17] Carbetocin mimics this mechanism.[23] Binding for carbetocin and other oxytocin agonists has been shown to be nonselective at the extracellular N-terminus and loops E2 and E3.[17] While the oxytocin receptor shows equal affinity for oxytocin and carbetocin, the biological effect of carbetocin is almost 50% that of endogenous or exogenous oxytocin.[23][17] Carbetocin has a much longer lasting effect than oxytocin, necessitating only a single dose. Carbetocin inhibits endogenous oxytocin release, interrupting the uterine feedback loop with the hypothalamus and decreasing both central and peripheral release of oxytocin.[22] Carbetocin is a biased agonist of the oxytocin receptor.[24]
During pregnancy, the synthesis of oxytocin receptors in the uterus greatly increases, reaching a peak during labor and delivery. Consequently, the administration of carbetocin or another oxytocin analog during or immediately following birth will have increased uterotonic and contractile effect. The application of carbetocin does not affect a non-pregnant uterus with lower oxytocin receptor expression.[10] Carbetocin also functions to thicken the blood, further preventing post-partum hemorrhage.[19] Carbetocin should not be used to induce or augment labor since it could cause cardiac or respiratory distress to mother or infant.[9][10]
Pharmacokinetics
Carbetocin is to be used in the hospital by prescription only. It can be administered intravenously or intramuscularly. In both cases, the recommended dose for an average adult female is 100 ug. Contractile effects of the uterus are apparent within two minutes and can be observed for approximately one hour,[18] though maximum binding occurs about 30 minutes after intramuscular injection. Administration is performed immediately following parturition to minimize risk of postpartum hemorrhage by inducing uterine contractions, increasing muscle tone and thickening the blood. If further uterine stimulation is needed, treatment with other forms of oxytocic uterotonic drugs should be used.[18]
Endogenous and synthetic oxytocin has a half-life of approximately 3.5 minutes.[10][23] Carbetocin, in comparison, has a much longer half-life ranging from 85 to 100 minutes.[10][23] The bioavailable dose is around 80%.[11] The elimination half-life following intravenous administration is around 40 minutes, though the elimination mechanism is not entirely known.[18] Studies have shown that elimination is only minimally renal (0.7%), but may occur at least partially through enzymatic degradation of peptides, primarily on the C-terminal end.[23] Both elimination and volume of distribution are not dose dependent.[18]
Society and culture
Legal approval
Carbetocin has been approved for use under the following three brand names in 23 countries, not including the United States: Duratocin (Argentina, Australia, Bahrain, Canada, China, Hong Kong, Italy, Malaysia, Singapore, New Zealand), Lonactene (Mexico), and Pabal (Austria, Belgium, Switzerland, Germany, Estonia, France, UK, Hungary, Lithuania, Luxembourg, Finland). Duratocin has also been approved for veterinary use in Poland, Germany, Italy, Belgium, Luxembourg, France and the Netherlands.[19]
Brand names
Duratocin, Pabal, Lonactene, Depotocin, Comoton, and Decomoton.
References
- Idan Shalev; Richard Paul Ebstein (2015). Social Hormones and Human Behavior: What Do We Know and Where Do We Go from Here. Frontiers Media SA. pp. 51–. ISBN 978-2-88919-407-0.
- British national formulary : BNF 76 (76 ed.). Pharmaceutical Press. 2018. p. 804. ISBN 9780857113382.
- "PROPOSAL FOR INCLUSION OF CARBETOCIN IN THE WHO LIST OF ESSENTIAL MEDICINES" (PDF). WHO. Retrieved 25 October 2019.
- "PROPOSAL FOR INCLUSION OF CARBETOCIN IN THE WHO LIST OF ESSENTIAL MEDICINES" (PDF). WHO. Retrieved 12 November 2019.
- I.K. Morton; Judith M. Hall (2012). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. pp. 65–. ISBN 978-94-011-4439-1.
- J. Elks (2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 214–. ISBN 978-1-4757-2085-3.
- World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- "Carbetocin Drug Information, Professional". Drugs.com. Retrieved 12 November 2019.
- Attilakos, G; Psaroudakis, D; Ash, J; Buchanan, R; Winter, C; Donald, F; Hunt, LP; Draycott, T (July 2010). "Carbetocin versus oxytocin for the prevention of postpartum haemorrhage following caesarean section: the results of a double-blind randomised trial". BJOG: An International Journal of Obstetrics and Gynaecology. 117 (8): 929–36. doi:10.1111/j.1471-0528.2010.02585.x. PMID 20482535. S2CID 205616218.
- Moertl, MG; Friedrich, S; Kraschl, J; Wadsack, C; Lang, U; Schlembach, D (October 2011). "Haemodynamic effects of carbetocin and oxytocin given as intravenous bolus on women undergoing caesarean delivery: a randomised trial". BJOG: An International Journal of Obstetrics and Gynaecology. 118 (11): 1349–56. doi:10.1111/j.1471-0528.2011.03022.x. PMID 21668768. S2CID 41172212.
- Silcox, J; Schulz, P; Horbay, GL; Wassenaar, W (September 1993). "Transfer of carbetocin into human breast milk". Obstetrics and Gynecology. 82 (3): 456–9. PMID 8355953.
- Bajcsy, AC; Szenci, O; van der Weijden, GC; Doornenbal, A; Maassen, F; Bartyik, J; Taverne, MA (20 January 2006). "The effect of a single oxytocin or carbetocin treatment on uterine contractility in early postpartum dairy cows". Theriogenology. 65 (2): 400–14. doi:10.1016/j.theriogenology.2005.05.040. PMID 15993938.
- Schramme, AR; Pinto, CR; Davis, J; Whisnant, CS; Whitacre, MD (November 2008). "Pharmacokinetics of carbetocin, a long-acting oxytocin analogue, following intravenous administration in horses". Equine Veterinary Journal. 40 (7): 658–61. doi:10.2746/042516408X334343. PMID 19165935.
- Widmer M; et al. (2018), "Heat-stable carbetocin versus oxytocin to prevent hemorrhage after vaginal birth" (PDF), N Engl J Med, 379 (8): 743–752, doi:10.1056/NEJMoa1805489, PMID 29949473, S2CID 205103322.
- Malm M; et al. (2018), "Development and stability of a heat-stable formulation of carbetocin for the prevention of postpartum haemorrhage for use in low and middle-income countries", J Pept Sci, 24 (6): e3082, doi:10.1002/psc.3082, PMC 6001700, PMID 29700898.
- Mundasad, Smitha (28 June 2018). "Revamped drug could save lives of many new mothers: WHO". BBC News. Retrieved 28 June 2018.
- Gimpl, G; Postina, R; Fahrenholz, F; Reinheimer, T (7 March 2005). "Binding domains of the oxytocin receptor for the selective oxytocin receptor antagonist barusiban in comparison to the agonists oxytocin and carbetocin". European Journal of Pharmacology. 510 (1–2): 9–16. doi:10.1016/j.ejphar.2005.01.010. PMID 15740719.
- "Product Information - Duratocin". healthlinks.net. Archived from the original on 15 November 2011. Retrieved 5 June 2012.
- "Carbetocin". drugs.com. Archived from the original on 3 March 2016. Retrieved 5 June 2012.
- "Duratocin - Detailed Prescribing Information (Membership Required)". MIMS Malaysia. Archived from the original on 17 January 2014. Retrieved 5 June 2012.
- "Therapeutic Areas - Reproductive Health". Ferring Pharmaceuticals. Archived from the original on 25 May 2012. Retrieved 5 June 2012.
- Gimpl, G; Fahrenholz, F (April 2001). "The oxytocin receptor system: structure, function, and regulation". Physiological Reviews. 81 (2): 629–83. doi:10.1152/physrev.2001.81.2.629. PMID 11274341. S2CID 13265083.
- Engstrøm, T; Barth, T; Melin, P; Vilhardt, H (21 August 1998). "Oxytocin receptor binding and uterotonic activity of carbetocin and its metabolites following enzymatic degradation". European Journal of Pharmacology. 355 (2–3): 203–10. doi:10.1016/S0014-2999(98)00513-5. PMID 9760035.
- Gulliver D, Werry E, Reekie TA, Katte TA, Jorgensen W, Kassiou M (January 2019). "Targeting the Oxytocin System: New Pharmacotherapeutic Approaches". Trends Pharmacol Sci. 40 (1): 22–37. doi:10.1016/j.tips.2018.11.001. PMID 30509888. S2CID 54559394.
External links
- "Carbetocin". Drug Information Portal. U.S. National Library of Medicine.