Selepressin
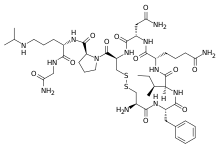
Selepressin (INN) (code name FE-202158), also known as [Phe(2),Ile(3), Hgn(4),Orn(iPr)(8)]vasopressin) is a potent, highly selective, short-acting peptide full agonist of the vasopressin 1A receptor and analog of vasopressin which was under development by Ferring Pharmaceuticals for the treatment of vasodilatory hypotension in septic shock.[1][2][3][4]
 | |
| Clinical data | |
|---|---|
| Other names | H-Cys(1)-Phe-Ile-hGln-Asn-Cys(1)-Pro-Orn(iPr)-Gly-NH2 |
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C46H73N13O11S2 |
| Molar mass | 1048.29 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
The Phase 2b/3 adaptive trial (SEPSIS-ACT) was terminated in February 2018 for futility. The trial was halted prior to the initiation of the arm for the highest dosing regimen of 5.0 ng/kg/min.[5]
References
- Levin JI (24 October 2014). Macrocycles in Drug Discovery. Royal Society of Chemistry. pp. 313–. ISBN 978-1-84973-701-2.
- Vincent J (23 September 2012). Annual Update in Intensive Care and Emergency Medicine 2012. Springer Science & Business Media. pp. 80–. ISBN 978-3-642-25716-2.
- Vincent JL (2 September 2008). Yearbook of Intensive Care and Emergency Medicine 2008. Springer Science & Business Media. pp. 427–. ISBN 978-3-540-77290-3.
- Laporte R, Kohan A, Heitzmann J, Wisniewska H, Toy J, La E, et al. (June 2011). "Pharmacological characterization of FE 202158, a novel, potent, selective, and short-acting peptidic vasopressin V1a receptor full agonist for the treatment of vasodilatory hypotension". The Journal of Pharmacology and Experimental Therapeutics. 337 (3): 786–96. doi:10.1124/jpet.111.178848. PMID 21411496. S2CID 576654.
- Clinical trial number NCT02508649 for "Selepressin Evaluation Programme for Sepsis-Induced Shock - Adaptive Clinical Trial" at ClinicalTrials.gov
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.