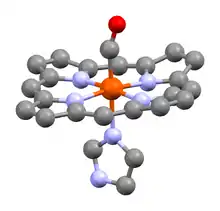
Carboxyhemoglobin
Carboxyhemoglobin (carboxyhaemoglobin BrE) (symbol COHb or HbCO) is a stable complex of carbon monoxide and hemoglobin (Hb) that forms in red blood cells upon contact with carbon monoxide. Carboxyhemoglobin is often mistaken for the compound formed by the combination of carbon dioxide (carboxyl) and hemoglobin, which is actually carbaminohemoglobin. Carboxyhemoglobin terminology emerged when carbon monoxide was known by its historic name, "carbonic oxide", and evolved through Germanic and British English etymological influences; the preferred IUPAC nomenclature is carbonylhemoglobin.[2][3][4]
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Carbonylhemoglobin | |||
| Other names
Carboxyhemoglobin Carboxyhaemoglobin Kohlenoxyhaemoglobin Kohlenoxyhämoglobin Kohlenoxydhämoglobin Kohlenmonoxyhämoglobin Carbonmonoxyhemoglobin Carbon-monoxide-hemoglobin Carbon-monoxide-Methemoglobin Carbonic oxide hæmoglobin | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |||
The average non-smoker maintains a systemic carboxyhemoglobin level under 3% COHb whereas smokers approach 10% COHb.[4] The biological threshold for carboxyhemoglobin tolerance is 15% COHb, meaning toxicity is consistently observed at levels in excess of this concentration.[5] The FDA has previously set a threshold of 14% COHb in certain clinical trials evaluating the therapeutic potential of carbon monoxide.[6]
Overview
The average red blood cell contains 250 million hemoglobin molecules.[7] Hemoglobin contains a globin protein unit with four prosthetic heme groups (hence the name heme -o- globin); each heme is capable of reversibly binding with one gaseous molecule (oxygen, carbon monoxide, cyanide, etc.),[8] therefore a typical red blood cell may carry up to one billion gas molecules. As the binding of carbon monoxide with hemoglobin is reversible, certain models have estimated that 20% of the carbon monoxide carried as carboxyhemoglobin may dissociate in remote tissues.[7]
Endogenous carbon monoxide production
In biology, carbon monoxide is naturally produced through many enzymatic and non-enzymatic pathways.[7] The most extensively studied pathway is the metabolism of heme by heme oxygenase which occurs throughout the body with significant activity in the spleen to facilitate hemoglobin breakdown during erythrocyte recycling. Therefore heme can both carry carbon monoxide in the case of carboxyhemoglobin, or, undergo enzymatic catabolism to generate carbon monoxide.
Carbon monoxide was characterized as a neurotransmitter in 1993 and has since been subcategorized as a gasotransmitter.[4]
Most endogenously produced carbon monoxide is stored as carboxyhemoglobin. The gas primarily undergoes pulmonary excretion, however trace amounts may be oxidized to carbon dioxide by certain cytochromes, metabolized by resident microbiota, or excreted by transdermal diffusion.[4][7]
Affinity of hemoglobin for carbon monoxide
Compared to oxygen, carbon monoxide binds with approximately 240 times greater affinity,[9][4] however the affinity of carbon monoxide for hemoglobin varies both across species and within a species. In the 1950s, Esther Killick was among the first to recognize a difference in carbon monoxide affinity between adult and foetal blood, and a difference between humans and sheep.[4][10][11] In humans, the Hb-Kirklareli mutation has a relative 80,000 times greater affinity for carbon monoxide than oxygen resulting in systemic carboxyhemoglobin reaching a sustained level of 16% COHb.[5] Other human mutations have been described (see also: hemoglobin variants).[12][13] Structural variations and mutations across other hemoproteins likewise affect carbon monoxide's interaction with the heme prosthetic group as exemplified by Cytochrome P450 where certain forms of the CYP3A family is relatively less affected by the inhibitory effects of carbon monoxide.[4]
Murinae species have a COHb half-life of 20 minutes compared to 300 minutes for a typical human (see toxicokinetics below).[4] As a result, the metabolic kinetics, blood saturation point, and tolerance for carbon monoxide exposure vary across species, potentially leading to data inconsistencies pertaining to the toxicology of carbon monoxide poisoning and pharmacology of low-dose therapeutic protocols.[4]
Some deep-diving marine mammal species are known to contain concentrations of carbon monoxide in their blood that resembles levels seen in chronic cigarette smokers, which may provide benefits against hypoxia.[14] Similarly, the elevated levels in smokers has been suggested to be a basis for the smoker's paradox.[4] Prolonged exposure to carbon monoxide and elevated carboxyhemoglobin, such as in smoking, results in erythremia.[4] Furthermore, humans can acclimate to toxic levels of carbon monoxide based on findings reported by Esther Killick.[4]
History
A bright red skin complexion is commonly associated with elevated carboxyhemoglobin levels. Trace evidence for an endogenous presence of carbon monoxide dates back to Marcellus Donato circa 1570 who noted an unusually red complexion upon conducting an autopsy of victims who died from charcoal fumes in Mantua.[4] Similar findings pertaining to red complexion later emerged as documented by Johann Jakob Wepfer in the 1600s, and M. Antoine Portal in the late 1700s.[4]
Phlogiston theory is a trace origin for the first chemical explanations of endogenous carboxyhemoglobin exemplified by the work of Joseph Priestley in the eighteenth century who suspected phlogiston to be a cellular waste product carried by the blood of animals which was subsequently exhaled.[4]
Thomas Beddoes, James Watt, Humphry Davy, James Lind, and many others investigated the therapeutic potential of inhaling factitious airs in the late eighteenth century (see also: Pneumatic Institution). Among the gases experimented with, hydrocarbonate had received significant attention. Hydrocarbonate is water gas generated by passing steam over coke, the process of which generates carbon monoxide and hydrogen, and some considered it contain phlogiston. Beddoes and Watt recognized hydrocarbonate brightened venous blood in 1793. Watt suggested coal fumes could act as an antidote to the oxygen in blood, and Beddoes and Watt likewise speculated hydrocarbonate has a greater affinity for animal fiber than oxygen in 1796.[4]
After the discovery of carbon monoxide by William Cruickshank in 1800, Johann Dömling (1803) and John Bostock (1804) developed hypotheses suggesting blood returned to the heart loaded with carbon monoxide to subsequently be oxidized to carbon dioxide in the lung prior to exhalation.[4] Later in 1854, Adrien Chenot similarly suggested carbon monoxide could remove oxygen from blood and be oxidized within the body to carbon dioxide.[4] The mechanism for carbon monoxide poisoning in the context of carboxyhemoglobin formation is widely credited to Claude Bernard whose memoirs beginning in 1846 and published in 1857 notably phrased, "prevents arterials blood from becoming venous".[4] Felix Hoppe-Seyler independently published similar conclusions in the following year.
The first analytical method to detect carboxyhemoglobin emerged in 1858 with a colorimetric method developed by Felix Hoppe-Seyler, and the first quantitative analysis method emerged in 1880 with Josef von Fodor.[4]
Etymology
Carbon is derived from the Latin term carbo, meaning coal, via the French charbone, which first appeared in print in 1786.[15] The etymology of oxygen is generally accepted mean 'acid' based on Lavoisier's system, which also recognized carbon as a nonmetallic element capable of oxidation, although the original degrees of oxides were based on diamond, graphite, coal and carbonic acid (CO2) as the most oxidized form;[15] Lavoisier's system was superseded by other obsolete oxide nomenclature systems.[16]
Upon discovering carbon monoxide through a series of experiments originating from coke (short for coal-cake[15]), Cruickshank named the new molecule "gaseous oxide of carbon" which evolved to "carbonic oxide" and was translated into German as "kohlenoxyd". Kohlen is the German word for coal.[4][17] As carbonic acid (CO2) was considered to be the most highly oxidized form in Lavoisier's system, the name carbonic oxide implied an intermediate oxidized species between coal and carbonic acid (i.e. use of the word acid indicated maximum oxidation).
Haem is derived from Greek meaning blood,[18][19] and globin is Latin derived from globus typically accepted to mean glob/spherical/round object; the terms are conjoined with an -o- . Regarding haem, the use of "ae / æ" remains prevalent in British English in modern day[20] whereas the American English spelling evolved to heme from hema.[19]
Felix Hoppe-Seyler coined the name "hämoglobin" in 1864.[21] In German, an umlaut such as ä is synonymous with spelling as "ae", therefore hämoglobin is commonly spelled as haemoglobin throughout German literature, hence haemoglobin is the term adopted by English literature.
Hoppe-Seyler likewise coined the name Kohlenoxydhämoglobin[22] which may have similarly been directly translated back into English as "carbonic oxide hæmoglobin".[23] The term carboxyhæmoglobin appeared as early as 1895 in works by John Haldane while the name for CO was still widely regarded as carbonic oxide.[24]
The term "carbon monoxide" was formally introduced in 1879, but the name would not become mainstream for several decades.[4] Variations of COHb terminology, such as carbonmonoxyhemoglobin,[25][11] followed and eventually evolved and simplified back into "carboxyhemoglobin".
As carboxy is now firmly associated with the CO2 carboxyl group, and carbon monoxide is generally regarded as a carbonyl, IUPAC has recommended "carbonylhemoglobin" as the preferred COHb nomenclature.[4] Despite the IUPAC guidance, carboxyhemoglobin remains the most widely used term (akin to the survival of bicarbonate nomenclature).
Analytical detection methods
Historically, carboxyhemoglobin detection has been achieved by colorimetric analysis, chemical reactivity, spectrophotometry, gasometric and thermoelectric detection methods.[4] Gas chromatography analysis emerged in 1961 and remains a commonly used method.[4]
Modern methods include pulse oximetry with a CO-oximeter, and a variety of other analytical techniques.[26][27] Most methods require laboratory equipment, skilled technicians, or expensive electronics therefore rapid and economical detection technologies remain in development.
Breath carbon monoxide is another detection method that may correlate with carboxyhemoglobin levels.[28]
Carbon monoxide poisoning
Carbon monoxide poisoning, also known as carboxyhemoglobinemia,[29][30] has plagued humankind since primitive ancestors first harnessed fire. In modern times, carboxyhemoglobin data assist physicians in making a poisoning diagnosis. However, carboxyhemoglobin levels do not necessarily correlate with the symptoms of carbon monoxide poisoning.[31] In general, 30% COHb is considered severe carbon monoxide poisoning.[4] The highest reported non-fatal carboxyhemoglobin level was 73% COHb.[4]
Mode of toxic action
Gas exchange is an essential process for many organisms to maintain homeostasis. Oxygen accounts for about 20% of Earth's atmospheric air. While inhaling air is critical to supply cells with oxygen for aerobic respiration via the Bohr effect and Haldane effect (and perhaps local low oxygen partial pressure e.g. active muscles),[32] exhaling the cellular waste product carbon dioxide is arguably the more critical aspect of respiration. Whereas the body can tolerate brief periods of hypoxia (as commonly occurs in anaerobic exercise, although the brain, heart, liver and kidney are significantly less tolerant than skeletal muscle), failure to expel carbon dioxide may cause respiratory acidosis (meaning bodily fluids and blood become too acidic thereby affecting homeostasis).[33] In absence of oxygen, cells switch to anaerobic respiration which if prolonged may significantly increase lactic acid leading to metabolic acidosis.[34]
To provide a simplified synopsis of the molecular mechanism of systemic gas exchange, upon inhalation of air it was widely thought oxygen binding to any of the heme sites triggers a conformational change in the protein unit of hemoglobin which then enables the binding of additional oxygen to each of the other heme sites. Upon arrival to the cellular region, oxygen is released at the tissue due to a conformational change in hemoglobin as caused by ionization of hemoglobin's surface due to the "acidification" of the tissue's local pH (meaning a relatively higher concentration of 'acidic' protons / hydrogen ions annotated as H+; an acidic pH is commonly referenced to as either low pH based on the acidity of pH 1-7 having a low number, or, referred to as a high pH due to the high concentration of H+ ions as the scale approaches pH 1); the local acidity is caused by an increase in the biotransformation of carbon dioxide waste into carbonic acid via carbonic anhydrase. In other words, oxygenated arterial blood arrives to cells in the "hemoglobin R-state" which has deprotonated/unionized amino acid residues (regarding hemoglobin's amines transitioning between the deprotonated/unionized Hb-NH2 to the protonated/ionized Hb-NH3+ state) based on the less-acidic pH (arterial blood averages pH 7.407 whereas venous blood is slightly more acidic at pH 7.371[35]). The "T-state" of hemoglobin is deoxygenated in venous blood partially due to protonation/ionization as caused by the acidic environment hence causing a conformation unsuited for oxygen-binding[36] (i.e. oxygen is 'ejected' upon arrival at the cell due to H+ ions bombarding the hemoglobin surface residues to convert Hb from "R-state" to "T-state"). Furthermore, the mechanism for formation of carbaminohemoglobin generates additional H+ ions that may further stabilize the protonated/ionized deoxygenated hemoglobin. Upon return of venous blood into the lung and subsequent exhalation of carbon dioxide, the blood is "de-acidified" (see also: hyperventilation) for the deprotonation/unionization of hemoglobin to re-enable oxygen binding as part of the transition to arterial blood (note this process is complex due to involvement of chemoreceptors, pH buffers and other physiochemical functionalities). Carbon monoxide poisoning disturbs this physiological process hence the venous blood of poisoning patients is bright red akin to arterial blood since the carbonyl/carbon monoxide is retained, whereas deoxygenated hemoglobin is dark red and carbaminohemoglobin has a blue hue.[13]
At toxic concentrations, carbon monoxide as carboxyhemoglobin significantly interferes with respiration and gas exchange by simultaneously inhibiting acquisition and delivery of oxygen to cells, and preventing formation of carbaminohemoglobin which accounts for approximately 30% of carbon dioxide exportation.[37] Therefore a patient suffering from carbon monoxide poisoning may experience severe hypoxia and acidosis in addition to the toxicities of excess carbon monoxide binding to numerous hemoproteins, metallic and non-metallic targets which affect cellular machinery (such as inhibition of cytochrome c oxidase).[7][38]
Toxicokinetics
In common air under normal atmospheric conditions, a typical patient's carboxyhemoglobin has a half-life around 300 minutes.[4] This time can be reduced to 90 minutes upon administration of high-flow pure oxygen, and the time is further reduced when oxygen is administered with 5% carbon dioxide as first identified by Esther Killick.[4] Additionally, treatment in a hyperbaric chamber is a more effective manner of reducing the half-life of carboxyhemoglobin to 30 minutes[4] and allows oxygen to dissolve in biological fluids for delivery to tissues.
Supplemental oxygen takes advantage of Le Chatelier's principle to quicken the decomposition of carboxyhemoglobin back to hemoglobin:[39]
- HbCO + O2 ⇌ Hb + CO + O2 ⇌ HbO2 + CO
Carboxyhemoglobin pharmaceuticals
As carbon monoxide is now understood to have a therapeutic potential, pharmaceutical efforts have focused on development of carbon monoxide-releasing molecules and selective heme oxygenase inducers.[40]
An alternative method for drug delivery consists of carbon monoxide immobilized on polyethylene glycol (PEG)-lyated bovine carboxyhemoglobin which is currently in late clinical development. Similarly, maleimide PEG conjugated human carboxyhemoglobin had previously been the subject of pharmaceutical development.[41]
See also
- Carbaminohemoglobin (Hb associated with CO2)
- Hemoglobinometer
- Hemoprotein
- Methemoglobin (ferric Hb, or ferrihemoglobin)
- Oxyhemoglobin (with diatomic oxygen, colored blood-red)
References
- Vásquez GB, Ji X, Fronticelli C, Gilliland GL (May 1998). "Human carboxyhemoglobin at 2.2 A resolution: structure and solvent comparisons of R-state, R2-state and T-state hemoglobins". Acta Crystallographica. Section D, Biological Crystallography. 54 (Pt 3): 355–366. doi:10.1107/S0907444997012250. PMID 9761903.
- "IUPAC Glossary of Terms Used in Toxicology - Terms Starting with C". www.nlm.nih.gov. Retrieved 2021-05-09.
- PubChem. "Carbon monoxide". pubchem.ncbi.nlm.nih.gov. Retrieved 2021-05-09.
- Hopper CP, Zambrana PN, Goebel U, Wollborn J (June 2021). "A brief history of carbon monoxide and its therapeutic origins". Nitric Oxide. 111: 45–63. doi:10.1016/j.niox.2021.04.001. PMID 33838343. S2CID 233205099.
- Motterlini R, Foresti R (March 2017). "Biological signaling by carbon monoxide and carbon monoxide-releasing molecules". American Journal of Physiology. Cell Physiology. 312 (3): C302–C313. doi:10.1152/ajpcell.00360.2016. PMID 28077358.
- Yang X, de Caestecker M, Otterbein LE, Wang B (July 2020). "Carbon monoxide: An emerging therapy for acute kidney injury". Medicinal Research Reviews. 40 (4): 1147–1177. doi:10.1002/med.21650. PMC 7280078. PMID 31820474.
- Hopper CP, De La Cruz LK, Lyles KV, Wareham LK, Gilbert JA, Eichenbaum Z, et al. (December 2020). "Role of Carbon Monoxide in Host-Gut Microbiome Communication". Chemical Reviews. 120 (24): 13273–13311. doi:10.1021/acs.chemrev.0c00586. PMID 33089988. S2CID 224824871.
- Berg JM, Tymoczko JL, Stryer L (2002). Biochemistry (5th ed.). New York: W H Freeman. ISBN 978-0-7167-3051-4.
- Berg JM, Tymoczko JL, Stryer L (2011). Biochemistry (7th ed.). New York: W H Freeman. ISBN 978-1-4292-7635-1.
- Stevenson, DK; Wong, RJ; Ostrander, CR; Maric, I; Vreman, HJ; Cohen, RS (April 2020). "Increased Carbon Monoxide Washout Rates in Newborn Infants". Neonatology. 117 (1): 118–122. doi:10.1159/000503635. ISSN 1661-7819. PMID 31634890. S2CID 204834990.
- Boor, AK (January 1930). "A Crystallographic Study Of Pure Carbonmon-Oxide Hemoglobin". The Journal of General Physiology. 13 (3): 307–316. doi:10.1085/jgp.13.3.307. ISSN 0022-1295. PMC 2141039. PMID 19872525.
- "Blood Discovery: New Hemoglobin Type Found". ScienceDaily. March 2008. Archived from the original on 2008-03-18. Retrieved 2021-10-27.
- "The Hemoglobin Page". East Tennessee State University. Archived from the original on 2004-09-19. Retrieved 2021-10-31.
- Tift, Michael S.; Alves de Souza, Rodrigo W.; Weber, Janick; Heinrich, Erica C.; Villafuerte, Francisco C.; Malhotra, Atul; Otterbein, Leo E.; Simonson, Tatum S. (2020). "Adaptive Potential of the Heme Oxygenase/Carbon Monoxide Pathway During Hypoxia". Frontiers in Physiology. 11: 886. doi:10.3389/fphys.2020.00886. ISSN 1664-042X. PMC 7387684. PMID 32792988.
- "History of Carbon". University of Kiel. Archived from the original on 2015-12-24. Retrieved 2021-10-31.
- Cooley, AJ (1845). A Cyclopaedia of Practical Receipts: And Collateral Information in the Arts, Manufacutres, and Trades, Including Medicine, Pharmacy, and Domestic Economy. John Churchill. pp. 647–648, 224.
- Coutts A (June 1959). "William Cruickshank of Woolwich". Annals of Science. 15 (2): 121–133. doi:10.1080/00033795900200118. ISSN 0003-3790.
- Meletis J (January 2002). "The derivatives of the Hellenic word "Haema" (hema, blood) in the English Language". Haema. 5 (2): 140–163 – via ResearchGate.
- Meletis J, Konstantopoulos K (2010). "The beliefs, myths, and reality surrounding the word hema (blood) from homer to the present". Anemia. 2010: 857657. doi:10.1155/2010/857657. PMC 3065807. PMID 21490910.
- Campbell NK, Fitzgerald HK, Dunne A (July 2021). "Regulation of inflammation by the antioxidant haem oxygenase 1". Nature Reviews. Immunology. 21 (7): 411–425. doi:10.1038/s41577-020-00491-x. PMID 33514947. S2CID 231762031.
- Westhorpe RN, Ball C (November 2008). "The pulse oximeter". Anaesthesia and Intensive Care. 36 (6): 767. doi:10.1177/0310057X0803600602. PMID 19115641. S2CID 44379880.
- Hoppe-Seyler F (1866). Medicinisch-chemische Untersuchungen: Aus dem Laboratorium für angewandte Chemie zu Tübingen (in German). A. Hirschwald. p. 119.
- Dobell H (January 1887). "On Asthma: Its Nature and Treatment". British Medical Journal. 1 (1360): 161–162. ISSN 0007-1447. PMC 2534062.
- Haldane, John (November 1895). "The Action of Carbonic Oxide on Man". The Journal of Physiology. 18 (5–6): 430–462. doi:10.1113/jphysiol.1895.sp000578. PMC 1514663. PMID 16992272.
- Pauling L, Coryell CD (April 1936). "The Magnetic Properties and Structure of Hemoglobin, Oxyhemoglobin and Carbonmonoxyhemoglobin". Proceedings of the National Academy of Sciences of the United States of America. 22 (4): 210–216. Bibcode:1936PNAS...22..210P. doi:10.1073/pnas.22.4.210. PMC 1076743. PMID 16577697.
- Vreman HJ, Wong RJ, Stevenson DK (2001). "Sources, sinks, and measurements of carbon monoxide.". Carbon monoxide and cardiovascular functions. CRC Press. pp. 273–307. doi:10.1201/9781420041019-23. ISBN 978-0-429-12262-0.
- Peng, H; Chen, W; Wang, B (July 2012). "Methods for the Detection of Gasotransmitters". In Hermann, A; Sitdikova, GF; Weiger, TM (eds.). Gasotransmitters: Physiology and Pathophysiology. Berlin, Heidelberg: Springer. pp. 99–137.
- Wald NJ, Idle M, Boreham J, Bailey A (May 1981). "Carbon monoxide in breath in relation to smoking and carboxyhaemoglobin levels". Thorax. 36 (5): 366–369. doi:10.1136/thx.36.5.366. PMC 471511. PMID 7314006.
- López-Herce J, Borrego R, Bustinza A, Carrillo A (September 2005). "Elevated carboxyhemoglobin associated with sodium nitroprusside treatment". Intensive Care Medicine. 31 (9): 1235–1238. doi:10.1007/s00134-005-2718-x. PMID 16041521. S2CID 10197279.
- Roth D, Hubmann N, Havel C, Herkner H, Schreiber W, Laggner A (June 2011). "Victim of carbon monoxide poisoning identified by carbon monoxide oximetry". The Journal of Emergency Medicine. 40 (6): 640–642. doi:10.1016/j.jemermed.2009.05.017. PMID 19615844.
- Hampson NB, Hauff NM (July 2008). "Carboxyhemoglobin levels in carbon monoxide poisoning: do they correlate with the clinical picture?". The American Journal of Emergency Medicine. 26 (6): 665–669. doi:10.1016/j.ajem.2007.10.005. PMID 18606318.
- Schmidt-Nielsen K (1997). Animal Physiology: Adaptation and Environment (Fifth ed.). Cambridge, UK: Cambridge University Press. ISBN 978-0-521-57098-5.
- "Respiratory acidosis: MedlinePlus Medical Encyclopedia". medlineplus.gov. Retrieved 2021-05-10.
- "Carbon Monoxide Poisoning" (PDF). ToxUpdate. Utah Poison Control Center. 6 (3): 1–3. 2004. Archived from the original (PDF) on 13 September 2015.
- O'Connor TM, Barry PJ, Jahangir A, Finn C, Buckley BM, El-Gammal A (2011). "Comparison of arterial and venous blood gases and the effects of analysis delay and air contamination on arterial samples in patients with chronic obstructive pulmonary disease and healthy controls". Respiration; International Review of Thoracic Diseases. 81 (1): 18–25. doi:10.1159/000281879. PMID 20134147.
- "Transport of Oxygen in the Blood" (PDF). Royal Society of Biology. Archived (PDF) from the original on 2020-11-28.
- Arthurs GJ, Sudhakar M (December 2005). "Carbon dioxide transport". Continuing Education in Anaesthesia, Critical Care & Pain. 5 (6): 207–210. doi:10.1093/bjaceaccp/mki050.
- Yang X, Lu W, Wang M, Tan C, Wang B (October 2021). ""CO in a pill": Towards oral delivery of carbon monoxide for therapeutic applications". Journal of Controlled Release. 338: 593–609. doi:10.1016/j.jconrel.2021.08.059. PMC 8526413. PMID 34481027.
- "ChemBytes: Week of February 8, 1998". www.columbia.edu. Retrieved 2021-05-11.
- Motterlini R, Otterbein LE (September 2010). "The therapeutic potential of carbon monoxide". Nature Reviews. Drug Discovery. 9 (9): 728–743. doi:10.1038/nrd3228. PMID 20811383. S2CID 205477130.
- Hopper CP, Meinel L, Steiger C, Otterbein LE (2018-05-31). "Where is the Clinical Breakthrough of Heme Oxygenase-1 / Carbon Monoxide Therapeutics?". Current Pharmaceutical Design. 24 (20): 2264–2282. doi:10.2174/1381612824666180723161811. PMID 30039755. S2CID 51712930.
External links
- Carboxyhemoglobin at the U.S. National Library of Medicine Medical Subject Headings (MeSH)