Cirsilineol
Cirsilineol is a bioactive flavone isolated from Artemisia[1] and from Teucrium gnaphalodes.[2]
 | |
| Names | |
|---|---|
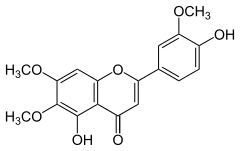
| IUPAC name
4′,5-Dihydroxy-3′,6,7-trimethoxyflavone | |
| Systematic IUPAC name
5-Hydroxy-2-(4-hydroxy-3-methoxyphenyl)-6,7-dimethoxy-4H-1-benzopyran-4-one | |
| Other names
Eupatrin | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C18H16O7 | |
| Molar mass | 344.319 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
References
- Sheng, X; Sun, Y; Yin, Y; Chen, T; Xu, Q (2008). "Cirsilineol inhibits proliferation of cancer cells by inducing apoptosis via mitochondrial pathway". The Journal of Pharmacy and Pharmacology. 60 (11): 1523–9. doi:10.1211/jpp/60.11.0014. PMID 18957174.
- Flavonoid Aglycones and Glycosides from Teucrium gnaphalodes. F. A. T. Barberán, M. I. Gil, F. Tomás, F. Ferreres and A. Arques, J. Nat. Prod., 1985, 48 (5), pages 859–860, doi:10.1021/np50041a040
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.