Cobalt(II) hydroxide
Cobalt(II) hydroxide or cobaltous hydroxide is the inorganic compound with the formula Co(OH)
2, consisting of divalent cobalt cations Co2+
and hydroxide anions OH−
. The pure compound, often called the "beta form" (β-Co(OH)
2) is a pink solid insoluble in water.[2][3]
 cobalt(II) hydroxide | |
| Names | |
|---|---|
| IUPAC name
Cobalt(II) hydroxide | |
| Other names
Cobaltous hydroxide, cobalt hydroxide, β-cobalt(II) hydroxide | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.040.136 |
| EC Number |
|
PubChem CID |
|
| UNII | |
| UN number | 3550 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| Co(OH)2 | |
| Molar mass | 92.948 g/mol |
| Appearance | rose-red powder or bluish-green powder |
| Density | 3.597 g/cm3 |
| Melting point | 168 °C (334 °F; 441 K) (decomposes)[1] |
| 3.20 mg/L | |
Solubility product (Ksp) |
1.0×10−15 |
| Solubility | soluble in acids, ammonia; insoluble in dilute alkalis |
| Structure | |
| rhombohedral | |
| Thermochemistry | |
Std molar entropy (S⦵298) |
79.0 J·mol−1·K−1[1] |
Std enthalpy of formation (ΔfH⦵298) |
-539.7 kJ·mol−1 |
| Hazards | |
| GHS labelling: | |
    | |
| Warning | |
| H302, H317, H319, H330, H334, H360, H372 | |
| P201, P202, P260, P261, P264, P270, P271, P272, P280, P281, P284, P285, P301+P312, P302+P352, P304+P340, P304+P341, P305+P351+P338, P308+P313, P310, P314, P320, P321, P330, P333+P313, P337+P313, P342+P311, P363, P403+P233, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Safety data sheet (SDS) | Oxford University |
| Related compounds | |
Other anions |
Cobalt(II) chloride Cobalt(II) bromide Cobalt(II) iodide |
Other cations |
Iron(II) hydroxide Nickel(II) hydroxide Copper(II) hydroxide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
The name is also applied to a related compound, often called "alpha" or "blue" form (α-Co(OH)
2), which incorporates other anions in its molecular structure. This compound is blue and rather unstable.[2][3]
Cobalt(II) hydroxide is most used as a drying agent for paints, varnishes, and inks, in the preparation of other cobalt compounds, as a catalyst and in the manufacture of battery electrodes.
Preparation
Cobalt(II) hydroxide precipitates as a solid when an alkali metal hydroxide is added to an aqueous solution of Co2+ salt.[4] For example,
- Co2+ + 2 NaOH → Co(OH)2 + 2 Na+
The compound can be prepared by reacting cobalt(II) nitrate in water with a solution of triethylamine N(C
2H
5)
3 as both the base and a complexing agent.[3] It can also be prepared by elecrolysis of a solution of cobalt nitrate with a platinum cathode.[5]
Reactions
Cobalt(II) hydroxide decomposes to cobalt(II) oxide at 168 °C under vacuum and is oxidized by air.[4] The thermal decomposition product in air above 300 °C is Co3O4.[6][7]
Like iron(II) hydroxide, cobalt(II) hydroxide is a basic hydroxide, and reacts with acids to form cobalt(II) salts. It also reacts with strong bases to form solutions with dark blue cobaltate(II) anions, [Co(OH)4]2− and [Co(OH)6]4−.[8]
Structure
The pure (β) form of cobalt(II) hydroxide has the brucite crystal structure. As such, the anion and cation packing are like those in cadmium iodide, in which the cobalt(II) cations have octahedral molecular geometry.[8]
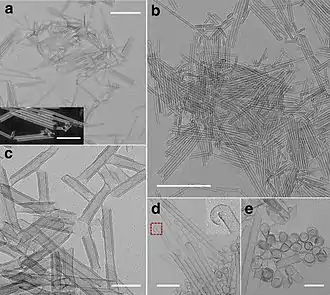
The beta form can be obtained as platelets with partial hexagonal geometry, 100-300 nm wide and 5-10 nm thick.[5][3]
Alpha form
The so-called "alpha form" (α-Co(OH)2) is not a polymorph of the pure (β) form, but rather a more complex compound in which hydroxide-cobalt-hydroxide layers have a residual positive charge and alternate with layers of other anions such as nitrate, carbonate, chloride, etc. (the hydrotalcite structure).[3] It is usually obtained as a blue precipitate when a base like sodium hydroxide is added to a solution of a cobalt(II) salt. The precipitate slowly converts to the beta form.[9]
Nanotubes
Cobalt hydroxide can be obtained in the form of nanotubes, which may be of interest in nanotechnology and materials science. [10]
References
- Lide, David R. (1998). Handbook of Chemistry and Physics (87 ed.). Boca Raton, Florida: CRC Press. p. 513. ISBN 0-8493-0594-2.
- Lide, David R. (1998). Handbook of Chemistry and Physics (87 ed.). Boca Raton, Florida: CRC Press. p. 454. ISBN 0-8493-0594-2.
- Xiaohe Liu, Ran Yi, Ning Zhang, Rongrong Shi, Xingguo Li, and Guanzhou Qiu (2008): "Cobalt hydroxide nanosheets and their thermal decomposition to cobalt oxide nanorings". Chemistry: An Asian Journal, volume 3, issue 4, pages 732-738. doi:10.1002/asia.200700264
- O. Glemser "Cobalt(II) Hydroxide" in Handbook of Preparative Inorganic Chemistry, 2nd Ed. Edited by G. Brauer, Academic Press, 1963, NY. Vol. 1. p. 1521.
- P. Benson, G. W. D. Briggs, and W. F. K. Wynne-Jones (1964): "The cobalt hydroxide electrode—I. Structure and phase transitions of the hydroxides". Electrochimica Acta, volume 9, issue 3, pages 275-280. doi:10.1016/0013-4686(64)80016-5
- Jayashree, R. S.; Kamath, P. Vishnu (1999). "Electrochemical synthesis of a-cobalt hydroxide". Journal of Materials Chemistry. 9 (4): 961–963. doi:10.1039/A807000H.
- Xu, Z. P.; Zeng, H. C. (1998). "Thermal evolution of cobalt hydroxides: a comparative study of their various structural phases". Journal of Materials Chemistry. 8 (11): 2499–2506. doi:10.1039/A804767G.
- Wiberg, Nils; Wiberg, Egon; Holleman, A. F. (2001). Inorganic Chemistry. Academic Press. pp. 1478–1479. ISBN 0-12-352651-5. Retrieved 2009-03-27.
- Liu, Zhaoping; Ma, Renzhi; Osada, Minoru; Takada, Kazunori; Sasaki, Takayoshi (2005). "Selective and Controlled Synthesis of α- and β-Cobalt Hydroxides in Highly Developed Hexagonal Platelets". Journal of the American Chemical Society. 127 (40): 13869–13874. doi:10.1021/ja0523338. PMID 16201808.
- Ni, Bing; Liu, Huiling; Wang, Peng-Peng; He, Jie; Wang, Xun (2015). "General synthesis of inorganic single-walled nanotubes". Nature Communications. 6: 8756. Bibcode:2015NatCo...6.8756N. doi:10.1038/ncomms9756. PMC 4640082. PMID 26510862.
