Conspecific song preference
Conspecific song preference is the ability songbirds require to distinguish conspecific song from heterospecific song in order for females to choose an appropriate mate, and for juvenile males to choose an appropriate song tutor during vocal learning. Researchers studying the swamp sparrow (Melospiza georgiana) have demonstrated that young birds are born with this ability, because juvenile males raised in acoustic isolation and tutored with artificial recordings choose to learn only songs that contain their own species' syllables.[1] Studies conducted at later life stages indicate that conspecific song preference is further refined and strengthened throughout development as a function of social experience.[2] The selective response properties of neurons in the songbird auditory pathway has been proposed as the mechanism responsible for both the innate and acquired components of this preference.
Neural mechanisms
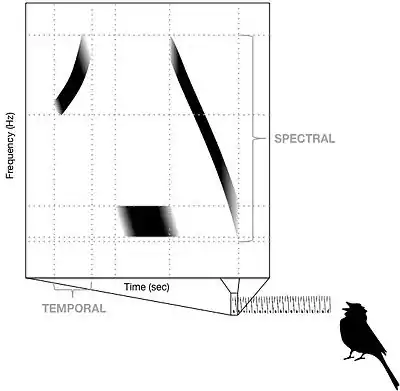
The mechanism responsible for the ability to distinguish song types has not yet been fully characterized by researchers in the field of neuroethology, but it has been demonstrated that at least five different structures within the auditory pathway contain neurons that preferentially respond to conspecific song. The structure of neural networks, the morphology of neurons, and the receptor and ion channel complement of pre-synaptic connections cause some neurons to respond maximally to a particular stimulus frequency, phase, amplitude or temporal pattern, and this is known as spectral-temporal tuning.[3] Tight spectral-temporal tuning in the auditory pathway provides the central nervous system of songbirds with the ability to discriminate between conspecific and heterospecific songs. Tuning characteristics of auditory neurons have been best characterized in zebra finch (Taeniopygia guttata), canary (Serinus canaria), European starling (Sturnus vulgaris) and barn owl (Tyto alba).
Learning and motor pathways
The conventional song system of songbirds has two parts: the anterior forebrain pathway (AFP) involved in song learning, and the posterior forebrain pathway or "song motor pathway" (PFP/SMP) involved in song production. Both of these descending pathways contain neurons that are responsive to conspecific song.[4] Female Canaries lost the ability to discriminate between conspecific and heterospecific song after bilateral lesions to the high vocal center HVC, a nucleus that sits at the apex of both pathways.[5] In males, however, most song system neurons respond maximally to the sound of the bird's own song, even more than they do to the tutor's song or any other conspecific song.[6][7] In HVC, neurons switch from responding best to tutor song (35–69 days post-hatch) to responding best to the bird's own song (>70 days post-hatch).[8] Song preferences of neurons in these pathways are important for sensorimotor learning, however several lines of evidence suggest that the specific ability to discriminate conspecific from heterospecific song does not reside in the AFP or the SMP. Most importantly, gene expression studies have demonstrated that, as a broad unit, neurons in the AFP and SMP show increased activation when a bird is singing, but not when it is simply listening to song.[9] With the exception of female Canaries, the neural substrate for conspecific song preference is thought to reside outside the conventional song system, in the auditory pathway.[10][11]
Cochlea
Avian hair cells have been extensively studied in the cochlea of the barn owl,[12] and it is now known that both the morphological structure of hair cell papillae and the ion channels that characterize hair cell membranes confer spectral tuning properties. Ca2+ dependent K+ channels are produced as splice variants of the cSlo gene,[13] and different isoforms cause the hair cell to preferentially respond to different resonant frequencies.[14][15] Species-specific differences in cSlo isoforms of hair cell membranes may therefore play a role in the discrimination of conspecific and heterospecific notes in songbirds.
Auditory thalamus
Studies on zebra finch have shown that nuclei in the auditory thalamus, two steps up from the cochlea, do not passively relay input from peripheral sensory structures into higher forebrain structures. Thalamic nuclei show different patterns of gene expression in response to different stimuli, implicating them in the process of acoustic discrimination.[16] Neurons in the nucleus ovoidalis (Ov) have receptive fields that are tuned to respond to the specific combination of spectral and temporal features present in syllables of conspecific song. Stimulus-selective tuning is determined by the receptor proteins and ion channels characterizing synapses of these neurons. Neurons can selectively respond to time-based differences between songs (e.g. syllable length or syllable-interval length) if they are post-synaptic to either fast-release (ionotropic) or slow-release (metabotropic) glutamate receptors.[17][18]
Auditory forebrain
Strong conspecific-selective responses have been most consistently demonstrated in neurons of the higher-level structures of the auditory system: The caudomedial neostriatum (NCM),[19][20] the auditory thalamo-recipient subfield (Field L: L1, L2a, L2b, L3),[2][21] and the caudal mesopallium (CM: CMM and CLM).[22][23][24][25] NCM and CM have been known to be discussed in conjunction with one another being as they are functionally similar. Recent studies have begun to show that while that is true, CM seems to respond in a manner relative to whether or not the stimulus is personally significant to the bird.
In European starlings, neurons in NCM habituate to a particular stimulus, and “remember” individual characteristics of songs to which a bird was exposed. This indicates that NCM functions in individual recognition, through the strategic recruitment of N-Methyl-D-aspartate receptors (NMDAR) to synapses that receive repeated patterns of excitation.[23][26] In fact, NMDARs are thought to be the unit broadly responsible for synaptic memory in the central nervous system. NMDARs in NCM neurons, therefore, might be a compelling target for selection when song functions in discrimination among conspecific songs, for neighbor recognition and territorial defense, but NCM is unlikely to play a role in the discrimination of conspecific from heterospecific songs.
In male zebra finches, neurons in Field L and CM do not exhibit a preference for different types of conspecific songs (in contrast to neurons in NCM, and those that participate in the AFP and SMP). Field L and CM neurons do not discriminate between the tutor song, the bird's own song, or individual conspecifics.[27] Instead, they demonstrate a higher-order preference for conspecific song over heterospecific song or other types of sound.[2][22] When male and female European starlings are trained to recognize conspecific song, there is an associated change in the response of CMM neurons,[28] and female zebra finches experience a reduced ability to discriminate between conspecific and heterospecific song following lesions to the region.[29] However, CMM neurons in females also show increased activation in response to their father's song over a novel conspecific song, demonstrating that this nucleus also participates in some selectivity among conspecific songs in females.[30][31][32]
Neurons in both Field L and CM have sophisticated filter properties, selective for both the spectral-temporal modulations and phase relationships of conspecific songs.[24] Furthermore, different neurons are selective for different features of syllables and songs. In Field L, neurons have one of four different tuning strategies—they are either tightly tuned a particular frequency, or they are sensitive to frequency edges, frequency sweeps or combined frequencies.[33][34] When exposed to natural song as a stimulus, different ensembles of these of neurons respond to different components of sound, and together they demonstrate the ability to perform sensitive discrimination between conspecific and heterospecific syllable types. As in nucleus ovoidalis, the spectral-temporal filter properties of Field L and CM neurons are a function of the particular ion channels and receptor proteins driving their synaptic dynamics. The complex and sophisticated tuning of these higher order processing centers for conspecific sounds may rely on the integrated inputs from the entire ascending auditory pathway, from the hair cells through the thalamus and forebrain, but this challenging synthetic question remains to be investigated.
Evolutionary significance
In an evolutionary context, neural mechanisms of conspecific song preference in the auditory pathway are important for species recognition. Species recognition traits play a central role in both the origin and maintenance of reproductive isolation. Furthermore, a shared neural mechanism for conspecific song preference has implications for the coevolution of male song and female preference, which may help explain the dramatic diversity of song phenotypes in extant songbirds<.
References
- Marler, P. and S. Peters. 1977. Selective vocal learning in a sparrow. Science 198:519-521.
- Amin, N., A. Doupe, and F. Theunissen. 2007. Development of selectivity for natural sounds in the songbird auditory forebrain. Journal of Neurophysiology 97:3517.
- Woolley, A.M.N., T.E. Fremouw, A. Hsu and F.E. Theunissen. 2005. Tuning for spectro-temporal modulations as a mechanism for auditory discrimination of natural sounds. Nature Neuroscience 8:1371-1379.
- Solis, M.M., M.S. Brainard, N.A. Hessler and A.J. Doupe. 2000. Song selectivity and sensorimotor signals in vocal learning and production. Proceedings of the National Academy of Sciences USA 97:11836-11842.
- Brenowitz, E.A. 1991. Altered perception of species-specific song by female birds after lesions of a forebrain nucleus. Science 251:303-304.
- Margoliash, D., and M. Konishi. 1985. Auditory representation of autogenous song in the song system of white-crowned sparrows. Proceedings of the National Academy of Sciences USA 82:5997-6000.
- Margoliash, D. 1986. Preference for autogenous song by auditory neurons in a song system nucleus of the white-crowned sparrow. Journal of Neuroscience 13:4737-4747.
- Nick, T.A., and M. Konishi. 2005. Neural song preference during vocal learning in the zebra finch depends on age and state. Journal of Neurobiology 62:231-242.
- Jarvis, E.D., and F. Nottebohm. 1997. Motor-driven gene expression. Proceedings of the National Academy of Sciences USA 94:4097-4102.
- Mello, C.V., D.S. Vicario and D.F. Clayton. 1992. Song presentation induces gene-expression in the songbird forebrain. Proceedings of the National Academy of Sciences 89:6818-6822.
- Bolhuis, J.J., G.G.O. Zijlstra, A.M. Den Boer-Visser and E.A. Van der Zee. 2000. Localized neuronal activation in the zebra finch brain is related to the strength of song learning. Proceedings of the National Academy of Sciences USA 97:2282-2285.
- Konishi, M., T.T. Takahashi, H. Wagner, W.E. Sullivan and C.E. Carr. 1988. Neurophysiological and anatomical substrates of sound localization in the owl. In “Auditory Function”. G.M. Edelman, W.E. Gall and W.M. Cowan, Eds. Wiley: New York.
- Rosenblatt, K.P., Z-P. Sun, S Heller and A.J. Hudspeth. 1997. Distribution of Ca2+-activated K+ channel isoforms along the tonotopic gradient of the chicken’s cochlea. Neuron 19:1061-1075.
- Koppl, C., G.A. Manley and M. Konishi. 2000. Auditory processing in birds. Current Opinion in Neurobiology 10:474-481.
- Fettiplace, R. and P.A. Fuchs. 1999. Mechanisms of hair cell tuning. Annual Review of Physiology 61:809-834.
- Brauth, S.E., W. Liang and W.S. Hall. 2006. Contact-call driven and tone-driven zenk expression in the nucleus ovoidalis of the budgerigar (Melopsittacus undulates). NeuroReport 17:1407-1410.
- Dutar, P., J. Petrozzino and H. Vu. 2000. Slow synaptic inhibition mediated by metabotropic glutamate receptor activation of GIRK channels. Journal of Neurophysiology 84:2284-2290.
- Amin, N., P. Gill and F. Theunissen. 2010. Role of the zebra finch auditory thalamus in generating complex representations for natural sounds. Journal of Neurophysiology 104:784.
- Bailey, D., and J. Rosebush. 2002. The hippocampus and caudomedial neostriatum show selective responsiveness to conspecific song in the female zebra finch. Journal of Neurobiology 52:43-51.
- George, I., H. Cousillas, and J. Richard. 2008. A potential neural substrate for processing functional classes of complex acoustic signals. PLoS ONE 3:e2203.
- Grace, J., N. Amin, and N. Singh. 2003. Selectivity for conspecific song in the zebra finch auditory forebrain. Journal of Neurophysiology 89:472-487.
- Boumans, T., C. Vignal and A. Smolders. 2008. Functional magnetic resonance imaging in zebra finch discerns the neural substrate involved in segregation of conspecific song from background noise. Journal of Neurophysiology 99:931-938.
- Chew, S., and D. Vicario. 1996. A large-capacity memory system that recognizes the calls and songs of individual birds. Proceedings of the National Academy of Sciences 93:1950-1955.
- Hsu, A., S. Woolley, T. Fremouw, and F. Theunissen. 2004. Modulation power and phase spectrum of natural sounds enhance neural encoding performed by single auditory neurons. The Journal of Neuroscience 24:9201.
- Gill, P., S. Woolley, T. Fremouw, and F. Theunissen. 2008. What's that sound? Auditory area CLM encodes stimulus surprise, not intensity or intensity changes. Journal of Neurophysiology 99:2809.
- George, I., H. Cousillas, and J. Richard. 2008. A potential neural substrate for processing functional classes of complex acoustic signals. PLoS ONE 3:e2203.
- Terpstra, N.J., J.J. Bolhuis and A.M. den Boer-Visser. 2004. An analysis of the neural representation of bird song memory. Journal of Neuroscience 24:4971-4977.
- Gentner, T.Q. and D. Margoliash. 2003. Neuronal populations and single cells representing learned auditory objects. Nature 424:669-674.
- MacDougall-Shackleton, S.A., S.H. Hulse and G.F. Ball. 1998. Neural bases of song preferences in female zebra finches (Taeniopygia guttata). NeuroReport 9:3047-3052.
- Terpstra, N.J., J.J. Bolhuis, K. Riebel, J.M.M. van der Burg and A.M. den Boer-Visser. 2006. Localised brain activation specific to auditory memory in a female songbird. Journal of Comparative Neurology 494:784-781.
- Wei, Jing; Liu, Quanxiao; Riebel, Katharina (2022-09-01). "Generalisation of early learned tutor song preferences in female zebra finches (Taeniopygia guttata)". Behavioural Processes. 201: 104731. doi:10.1016/j.beproc.2022.104731. ISSN 0376-6357.
- Miller, David B. (1979-08-01). "Long-term recognition of father's song by female zebra finches". Nature. 280 (5721): 389–391. doi:10.1038/280389a0. ISSN 1476-4687.
- Theunissen, F., K. Sen, and A. Doupe. 2000. Spectral-temporal receptive fields of nonlinear auditory neurons obtained using natural sounds. The Journal of Neuroscience 20:2315.
- Theunissen, F., S. David, N. Singh, A. Hsu, W. Vinje, and J. Gallant. 2001. Estimating spatio-temporal receptive fields of auditory and visual neurons from their responses to natural stimuli. Network: Computation in Neural Systems 12:289-316.
