Contractile vacuole
A contractile vacuole (CV) is a sub-cellular structure (organelle) involved in osmoregulation. It is found predominantly in protists and in unicellular algae. It was previously known as pulsatile or pulsating vacuole.
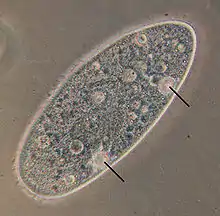
Overview
The contractile vacuole is a specialized type of vacuole that regulates the quantity of water inside a cell. In freshwater environments, the concentration of solutes is hypotonic, lower outside than inside the cell. Under these conditions, osmosis causes water to accumulate in the cell from the external environment. The contractile vacuole acts as part of a protective mechanism that prevents the cell from absorbing too much water and possibly lysing (rupturing) through excessive internal pressure.
The contractile vacuole, as its name suggests, expels water out of the cell by contracting. The growth (water gathering) and contraction (water expulsion) of the contractile vacuole are periodical. One cycle takes several seconds, depending on the species and the osmolarity of the environment. The stage in which water flows into the CV is called diastole. The contraction of the contractile vacuole and the expulsion of water out of the cell is called systole.
Water always flows first from outside the cell into the cytoplasm, and is only then moved from the cytoplasm into the contractile vacuole for expulsion. Species that possess a contractile vacuole typically always use the organelle, even at very hypertonic (high concentration of solutes) environments, since the cell tends to adjust its cytoplasm to become even more hyperosmotic than the environment. The amount of water expelled from the cell and the rate of contraction are related to the osmolarity of the environment. In hyperosmotic environments, less water will be expelled and the contraction cycle will be longer.
The best-understood contractile vacuoles belong to the protists Paramecium, Amoeba, Dictyostelium and Trypanosoma, and to a lesser extent the green alga Chlamydomonas. Not all species that possess a contractile vacuole are freshwater organisms; some marine, soil microorganisms and parasites also have a contractile vacuole. The contractile vacuole is predominant in species that do not have a cell wall, but there are exceptions (notably Chlamydomonas) which do possess a cell wall. Through evolution, the contractile vacuole has typically been lost in multicellular organisms, but it still exists in the unicellular stage of several multicellular fungi, as well as in several types of cells in sponges (amoebocytes, pinacocytes, and choanocytes).[1]
The number of contractile vacuoles per cell varies, depending on the species. Amoeba have one, Dictyostelium discoideum, Paramecium aurelia and Chlamydomonas reinhardtii have two, and giant amoeba, such as Chaos carolinensis, have many. The number of contractile vacuoles in each species is mostly constant and is therefore used for species characterization in systematics. The contractile vacuole has several structures attached to it in most cells, such as membrane folds, tubules, water tracts and small vesicles. These structures have been termed the spongiome; the contractile vacuole together with the spongiome is sometimes called the "contractile vacuole complex" (CVC). The spongiome serves several functions in water transport into the contractile vacuole and in localization and docking of the contractile vacuole within the cell.
Paramecium and Amoeba possess large contractile vacuoles (average diameter of 13 and 45 µm, respectively), which are relatively comfortable to isolate, manipulate and assay. The smallest known contractile vacuoles belong to Chlamydomonas, with a diameter of 1.5 µm. In Paramecium, which has one of the most complex contractile vacuoles, the vacuole is surrounded by several canals, which absorb water by osmosis from the cytoplasm. After the canals fill with water, the water is pumped into the vacuole. When the vacuole is full, it expels the water through a pore in the cytoplasm which can be opened and closed.[2] Other protists, such as Amoeba, have CVs that move to the surface of the cell when full and undergo exocytosis. In Amoeba contractile vacuoles collect excretory waste, such as ammonia, from the intracellular fluid by both diffusion and active transport.
Water flow into the CV
The way in which water enters the CV had been a mystery for many years, but several discoveries since the 1990s have improved understanding of this issue. Water could theoretically cross the CV membrane by osmosis, but only if the inside of the CV is hyperosmotic (higher solute concentration) to the cytoplasm. The discovery of proton pumps in the CV membrane[3] and the direct measurement of ion concentrations inside the CV using microelectrodes[4] led to the following model: the pumping of protons either into or out of the CV causes different ions to enter the CV. For example, some proton pumps work as cation exchangers, whereby a proton is pumped out of the CV and a cation is pumped at the same time into the CV. In other cases, protons pumped into the CV drag anions with them (carbonate, for example), to balance the pH. This ion flux into the CV causes an increase in CV osmolarity and as a result water enters the CV by osmosis. Water has been shown in at least some species to enter the CV through aquaporins.[5]
Acidocalcisomes have been implied to work alongside the contractile vacuole in responding to osmotic stress. They were detected in the vicinity of the vacuole in Trypanosoma cruzi and were shown to fuse with the vacuole when the cells were exposed to osmotic stress. Presumably the acidocalcisomes empty their ion contents into the contractile vacuole, thereby increasing the vacuole's osmolarity.[6]
Unresolved issues
The CV does not exist in higher organisms, but some of its unique characteristics are used by them in their osmoregulatory mechanisms. Research on the CV can therefore help us understand how osmoregulation works in all species. Many issues regarding the CV remain, as of 2010, unsolved:
- Contraction. It is not completely known what causes the CV membrane to contract, and whether it is an active process which costs energy or a passive collapse of the CV membrane. Evidence for involvement of actin and myosin, prominent contractile proteins which are found in many cells, are ambiguous.
- Membrane composition. Although it is known that several proteins decorate the CV membrane (V−H+−ATPases, aquaporins), a complete list is missing. The composition of the membrane itself and its similarities to and differences from other cellular membranes are also not clear.
- Contents of the CV. Several studies have shown the ion concentrations inside some of the largest CVs but not in the smallest ones (such as in the important model organism Chlamydomonas rheinhardii). The reasons and mechanisms for ion exchange between the CV and cytoplasm are not entirely clear.
References
- Brauer EB, McKanna JA (1978). "Contractile vacuoles in cells of a freshwater sponge, Spongilla Lacustris". Cell Tissue Res. 192 (2): 309–317. doi:10.1007/bf00220748. PMID 699019.
- Allen RD (2000). "The contractile vacuole and its membrane dynamics". BioEssays. 22 (11): 1035–1042. doi:10.1002/1521-1878(200011)22:11<1035::AID-BIES10>3.0.CO;2-A. PMID 11056480.
- Heuser J, Zhu Q, Clarke M (1993). "Proton pumps populate the contractile vacuoles of Dictyostelium amoebae". J Cell Biol. 121 (6): 1311–1327. doi:10.1083/jcb.121.6.1311. PMC 2119701. PMID 8509452.
- Stock C, Gronlien HK, Allen RD, Naitoh Y (2002). "Osmoregulation in Paramecium: in situ ion gradients permit water to cascade through the cytosol to the contractile vacuole". J Cell Sci. 115 (Pt 11): 2339–2348. PMID 12006618.
- Nishihara E, Yokota E, Tazaki A, Orii H, Katsuhara M, Kataoka K, Igarashi H, Moriyama Y, Shimmen T, Sonobe S (2008). "Presence of aquaporin and V-ATPase on the contractile vacuole of Amoeba proteus". Biol Cell. 100 (3): 179–188. doi:10.1042/BC20070091. PMID 18004980.
- Rohloff P, Montalvetti A, Docampo R (2004). "Acidocalcisomes and the Contractile Vacuole Complex Are Involved in Osmoregulation in Trypanosoma cruzi". J Biol Chem. 279 (50): 52270–52281. doi:10.1074/jbc.M410372200. PMID 15466463.