Paris green
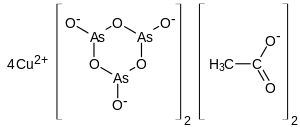
Paris green (copper(II) acetate triarsenite or copper(II) acetoarsenite) is an arsenic-based organic pigment. As a green pigment it is also known as Schweinfurt green, emerald or Vienna green. It is a highly toxic emerald-green crystalline powder[4] that has been used as a rodenticide and insecticide,[5] and also as a pigment. It was manufactured in 1814 to be a pigment to make a vibrant green paint, and was used by many notable painters in the 19th century. The color of Paris green is said to range from a pale blue green when very finely ground, to a deeper green when coarsely ground. Due to the presence of arsenic, the pigment is extremely toxic and in paintings, the color can degrade quickly.
 | |
| Names | |
|---|---|
| Other names
C.I. pigment green 21, emerald green, Schweinfurt green, imperial green, Vienna green, Mitis green, Veronese green[1] | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.125.242 |
| EC Number |
|
PubChem CID |
|
| UNII | |
| UN number | 1585 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| Cu(C2H3O2)2·3Cu(AsO2)2 | |
| Molar mass | 1013.79444 g/mol |
| Appearance | Emerald green crystalline powder |
| Density | >1.1 g/cm3 (20 °C) |
| Melting point | > 345 °C (653 °F; 618 K) |
| Boiling point | decomposes |
| insoluble | |
| Solubility | soluble but unstable in acids insoluble in alcohol |
| Hazards | |
| GHS labelling: | |
  | |
| Danger | |
| H302, H410 | |
| P260, P264, P273, P280, P301+P312, P301+P330+P331, P303+P361+P353, P304+P340, P305+P351+P338, P310, P362, P391, P405, P501 | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
22 mg/kg |
| NIOSH (US health exposure limits): | |
PEL (Permissible) |
[1910.1018] TWA 0.010 mg/m3[2] |
REL (Recommended) |
Ca C 0.002 mg/m3 [15-minute][2] |
IDLH (Immediate danger) |
Ca [5 mg/m3 (as As)][2] |
| Safety data sheet (SDS) | CAMEO MSDS |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
| Paris green | |
|---|---|
| Hex triplet | #50C878 |
| sRGBB (r, g, b) | (80, 200, 120) |
| HSV (h, s, v) | (140°, 60%, 78%) |
| CIELChuv (L, C, h) | (72, 71, 137°) |
| Source | Maerz and Paul[3] |
| ISCC–NBS descriptor | Vivid yellowish green |
| B: Normalized to [0–255] (byte) | |
Preparation and structure
Paris green may be prepared by combining copper(II) acetate and arsenic trioxide.[6] The structure was confirmed by X-ray crystallography.[7]
_(ASOCUA).png.webp)
History
In 1814, Paris green was invented by paint manufacturers, Wilhelm Sattler and Friedrich Russ, in Schweinfurt, Germany for the Wilhelm Dye and White Lead Company. They were attempting to produce a more stable pigment than Scheele's green, seeking to make a green that was less susceptible to darkening around sulfides.[lower-roman 1] In 1822, the recipe for emerald green was published by Justus von Liebig and André Braconnot.[8] In 1867, the pigment was named Paris green and was officially recognized as the first chemical insecticide in the world. Because of its arsenic content, the pigment was dangerous and toxic to manufacture, often resulting in factory poisonings.[9][10] At the time, emerald green was praised as a more durable and vibrant substitute for Scheele’s green, even though it would later prove to degrade quickly and react with other manufactured paints.
Pigment
In paintings, the pigment produces a rich, dark green with an undertone of blue. In comparison, Scheele's green is more yellow, and therefore, more lime-green.[11]: 220 Paris green became popular in the 19th century because of its brilliant color.[11]: 223 It was also called Emerald green because of its resemblance to the gemstone's deep color.
Notable occurrences
Paris green was widely used by 19th-century artists. It is present in several paintings by Claude Monet and Paul Gauguin, who found its color difficult to replicate with natural materials.[11]: 256
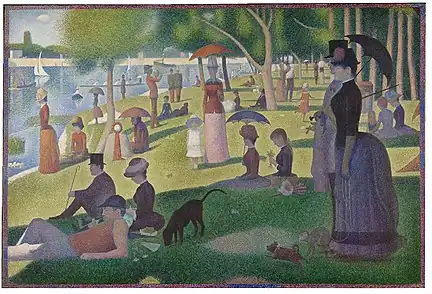
Permanence
.JPG.webp)
The pigment has a tendency to darken and turn brown. The issue was already apparent in the 19th century. In a 1888 study, watercolors with the pigment were shown to darken and turn brown when exposed to natural light and air. Experiments at the turn of the 20th century gave mixed results. Some found that the Paris green degraded slightly while other sources said the pigment was weatherproof.[11]: 227 This discrepancy could be due to the fact that each experiment used a different brand of Paris green.[11]: 228
Paris green in Descente des Vaches by Théodore Rousseau has changed significantly.[12]
Related pigments
Similar natural compounds are the minerals chalcophyllite Cu
18Al
2(AsO
4)
3(SO
4)
3(OH)
27·36H
2O, conichalcite CaCu(AsO
4)(OH), cornubite Cu
5(AsO
4)
2(OH)
4·H
2O, cornwallite Cu
5(AsO
4)
2(OH)
4·H
2O, and liroconite Cu
2Al(AsO
4)(OH)
4·4H
2O. These minerals range in color from greenish blue to slightly yellowish green.
Scheele's green is a chemically simpler, less brilliant, and less permanent, copper-arsenic pigment used for a rather short time before Paris green was first prepared, which was approximately 1814. It was popular as a wallpaper pigment and would degrade, with moisture and molds, to arsine gas. Paris green was also used in wallpaper to some extent and may have also degraded similarly.[13] Both pigments were once used in printing ink formulations.
The ancient Romans used one of them, possibly conichalcite, as a green pigment. The Paris green paint used by the Impressionists is said to have been composed of relatively coarse particles. Later, the chemical was produced with increasingly small grinds and without carefully removing impurities; its permanence suffered. It is likely that it was ground more finely for use in watercolors and inks, too.
Insecticide
In 1867, farmers in Illinois and Indiana found that Paris green was effective against the Colorado potato beetle, an aggressive agricultural pest. Despite concerns regarding the safety of using arsenic compounds on food crops, Paris green became the preferred method for controlling the beetle. By the 1880s, Paris green had become the first widespread use of a chemical insecticide in the world.[14] It was also used widely in the Americas to control the tobacco budworm, Heliothis virescens.[15]
Paris green was heavily sprayed by airplane in Italy, Sardinia, and Corsica during 1944 and in Italy in 1945 to control malaria.[16] It was once used to kill rats in Parisian sewers, which is how it acquired its common name.[17]
However, the manufacturing of the insecticide caused many health complications for factory workers, and in certain cases was lethal.[18]
 Mixing "Paris green" and road dust preparatory to dusting streams and breeding places of mosquitoes during World War II
Mixing "Paris green" and road dust preparatory to dusting streams and breeding places of mosquitoes during World War II Use as insecticide, poster issued by US Public Health Service
Use as insecticide, poster issued by US Public Health Service
See also
References
- Sulfur was commonly produced from burning coal fires.
- "Health & Safety in the Arts -- Painting & Drawing Pigments". Environmental Management Division, City of Tucson AZ. Archived from the original on 20 July 2011. Retrieved 7 February 2011.
- NIOSH Pocket Guide to Chemical Hazards. "#0038". National Institute for Occupational Safety and Health (NIOSH).
- The color displayed in the color box above matches the color called emerald green in the 1930 book by Maerz and Paul A Dictionary of Color New York:1930 McGraw-Hill; the color emerald green is displayed on page 75, Plate 26, Color Sample J10.
- "Hazardous Substance Fact Sheet" (PDF). NJ Dept. of Health and Senior Services. Retrieved 7 February 2011.
- "Dangers in the Manufacture of Paris Green and Scheele's Green". Monthly Review of the U.S. Bureau of Labor Statistics. 5 (2): 78–83. 1917. JSTOR 41829377.
- "H.Wayne Richardson, "Copper Compounds" in Ullmann's Encyclopedia of Industrial Chemistry 2005, Wiley-VCH, Weinheim. doi:10.1002/14356007.a07_567
- Pertlik, F. (1977). "Die Kristallstruktur von Cu2As3O6CH3COO". Zeitschrift für Kristallographie. 145 (1–2): 35–45. Bibcode:1977ZK....145...35P. doi:10.1524/zkri.1977.145.1-2.35.
- Zieske, Faith (1995). "An Investigation of Paul Cézanne's Watercolors With Emphasis on Emerald". The Book and Paper Group: Annual.
- Haynes, William (1954). American Chemical Industry: Background and Beginnings (Vol. 1 ed.). D. Van Nostrand Company, Inc. pp. 355–369.
- Emsley, John (2005). The Elements of Murder: A History of Poison. OUP. p. 118. ISBN 9780192805997.
- Fiedler, Inge; Bayard, Michael A. (2012). "Emerald Green and Scheele's Green". Artists' Pigments: A Handbook of Their History and Characteristics. Vol. 3. Washington D.C.: National Gallery of Art. pp. 219–71.
- Keune, Katrien; Boon, Jaap J.; Boitelle, R.; Shimazdu, Y. (July 2013). "Degradation of Emerald Green in Oil Paint and Its Contribution to the Rapid Change in Colour of the Descente Des Vaches (1834-1835)". Studies in Conservation. 58 (3): 199–210 – via JSTOR.
- Zawacki, Alexander J. (23 January 2018). "How a Library Handles a Rare and Deadly Book of Wallpaper Samples". Atlas Obscura.
- Sorenson 1995
- Blanco, Carlos (2012). "Heliothis virescens and Bt cotton in the United States". GM Crops & Food: Biotechnology in Agriculture and the Food Chain. 3 (3): 201–212. doi:10.4161/gmcr.21439. PMID 22892654.
- Justin M. Andrews, Sc. D. (1963). "Preventive Medicine in World War II, Chapter V. North Africa, Italy, and the Islands of the Mediterranean". Washington, D.C. USA: Office of the Surgeon General, Department of the Army. p. 281. Retrieved 30 September 2008.
- The Natural Paint Book, by Lynn Edwards, Julia Lawless, Table of contents
- Whorton, James C. (2010). The Arsenic Century: How Victorian Britain was Poisoned at Home, Work, and Play. OUP. p. 162. ISBN 9780191623431.
Further reading
- Fiedler, I. and Bayard, M. A., "Emerald Green and Scheele’s Green", in Artists' Pigments: A Handbook of Their History and Characteristics, Vol. 3: E.W. Fitzhugh (Ed.) Oxford University Press 1997, pp. 219–271
- Hughes, Michael F.; et al. (2011). "Arsenic Exposure and Toxicology: A Historical Perspective". Toxicological Sciences. 123 (2): 305–332. doi:10.1093/toxsci/kfr184. PMC 3179678. PMID 21750349.
- Sorensen, W. Conner (1995). Brethren of the Net, American Entomology, 1840-1880. University of Alabama Press. pp. 124–125.
- Spear, Robert J., The Great Gypsy Moth War, A History of the First Campaign in Massachusetts to Eradicate the Gypsy Moth, 1890–1901. University of Massachusetts Press, Amherst and Boston, 2005. ISBN 1-55849-479-0