Deinococcus
Deinococcus (from the Greek: δεινός, deinos, "dreadful, strange" and κόκκος, kókkos, "granule"[1]) is in the monotypic family Deinococcaceae, and one genus[2] of three in the order Deinococcales[3][4] of the bacterial phylum Deinococcota highly resistant to environmental hazards. These bacteria have thick cell walls that give them Gram-positive stains, but they include a second membrane and so are closer in structure to Gram-negative bacteria. Deinococcus survive when their DNA is exposed to high doses of gamma and UV radiation. Whereas other bacteria change their structure in the presence of radiation, such as by forming endospores, Deinococcus tolerate it without changing their cellular form and do not retreat into a hardened structure. They are also characterized by the presence of the carotenoid pigment deinoxanthin that give them their pink color. They are usually isolated according to these two criteria. In August 2020, scientists reported that bacteria from Earth, particularly Deinococcus bacteria, were found to survive for three years in outer space, based on studies conducted on the International Space Station. These findings support the notion of panspermia, the hypothesis that life exists throughout the Universe, distributed in various ways, including space dust, meteoroids, asteroids, comets, planetoids or contaminated spacecraft.[5][6]
| Deinococcus | |
|---|---|
 | |
| A tetrad of D. radiodurans | |
| Scientific classification | |
| Domain: | Bacteria |
| Phylum: | Deinococcota |
| Class: | Deinococci |
| Order: | Deinococcales |
| Family: | Deinococcaceae Brooks and Murray 1981 |
| Genus: | Deinococcus Rainey et al. 1997 |
| Type species | |
| Deinococcus radiodurans Raj et al. 1960 ex Brooks and Murray 1981 | |
| Synonyms | |
| |
Molecular signatures
Members of Deinococcus can be distinguished from all other bacteria through molecular signatures known as conserved signature indels (CSIs) and proteins (CSPs). An earlier study on Deinococcus identified nine CSIs and 58 CSPs which were exclusively shared by members of this genus.[7] Some of the identified CSPs such as the DNA damage repair protein PprA and the single-stranded DNA-binding protein DdrB are thought to have functional roles in the DNA repair mechanism and radioresistance phenotype of Deinococcus.[7]
In a more recent work focused on DNA repair proteins an additional 22 CSIs were identified as specific to this genus, including a 30 amino acid insert in the UvrA1 protein that is suggested to play in a role in the resistance ability of Deinococcus species against radiation and oxidation damage.[8]
The uvrA1 gene in Deinococcus was found to form a novel genetic linkage with the genes of the proteins dCSP-1 (a transmembrane protein found only in Deinococcus species), DsbA and DsbB. The latter two proteins play a central role in the formation of disulfide bonds in proteins via oxidation-reduction of cysteine rich motifs (CXXC).[9] The above cluster of genes forms a novel operon unique to Deinococcus species and the encoded proteins are predicted to function together to combat against DNA damage caused by reactive oxidative species from radiation.[8]
The 30 aa CSI present in UvrA1 and another 5-7 aa CSI present in DsbA are located on surface loops of the proteins. The surface exposed loops/patches formed by these CSIs are thought to mediate protein-protein interactions with the transmembrane protein dCSP-1, thus facilitating a sequence of electron transfers that ultimately ameliorates oxidative damage.[8]
Taxonomy
The currently accepted taxonomy is based on the List of Prokaryotic names with Standing in Nomenclature (LPSN) [10] and National Center for Biotechnology Information (NCBI).[11] As of August 2011, there were 47 species of Deinococcus described
Unassigned species:
- "D. aestuarii" Yin et al. 2022
- "D. aquivivus" Kaempferet al. 2008
- "D. gammatolerans" Srinivasan, Kang & Kim 2017
- "D. guangxiensis" Sun et al. 2009
- "D. koreense" Kim, Kang & Srinivasan 2017
- "D. populi" Li, Kudo & Tonouchi 2018
- "D. sahariens" Bouraoui et al. 2012
- "D. soli" Zhang et al. 2011 non Cha et al. 2016
- "D. xianganensis" Zheng et al. 2014
- D. xibeiensis Wang et al. 2010[12]
Comparative genomics
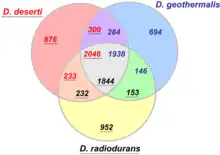
Although all species of the genus Deinococcus are related by definition, they exhibit substantial differences across their genomes. Most species appear to have about 3,000 genes, but only a fraction of them are shared in other species. For example, a 3-species comparison among D. radiodurans, D. deserti, and D. geothermalis shows that about two thirds of each genome is shared by all three species, but close to a third is specific and only found in one of the species (see figure). Once more genomes are included in such comparisons, the core genome will almost certainly be much smaller.[13]
Phylogeny
| 16S rRNA based LTP_01_2022[14][15][16] | 120 marker proteins based GTDB 07-RS207[17][18][19] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
References
- Deinococcus entry in LPSN; Euzéby, J.P. (1997). "List of Bacterial Names with Standing in Nomenclature: a folder available on the Internet". International Journal of Systematic and Evolutionary Microbiology. 47 (2): 590–2. doi:10.1099/00207713-47-2-590. PMID 9103655.
- Brooks BW, Murray RGE (1981) Nomenclature for" Micrococcus radiodurans" and other radiation-resistant cocci: Deinococcaceae fam. nov. and Deinococcus gen. nov., including five species. International Journal of Systematic and Evolutionary Microbiology 31: 353.
- Ekman JV, Raulio M, Busse HJ, Fewer DP, Salkinoja-Salonen M (2010) Deinobacterium chartae gen. nov., sp. nov., an extremely radiation resistant biofilm-forming bacterium isolated from a Finnish paper mill. International Journal of Systematic and Evolutionary Microbiology.
- Albuquerque L, Sims C, Nobre MF, Pino NM, Battista JR, et al. (2005) Truepera radiovictrix gen. nov., sp. nov., a new radiation-resistant species and the proposal of Trueperaceae fam. nov. FEMS Microbiology Letters 247: 161-169.
- Strickland, Ashley (26 August 2020). "Bacteria from Earth can survive in space and could endure the trip to Mars, according to new study". CNN News. Retrieved 26 August 2020.
- Kawaguchi, Yuko; et al. (26 August 2020). "DNA Damage and Survival Time Course of Deinococcal Cell Pellets During 3 Years of Exposure to Outer Space". Frontiers in Microbiology. 11: 2050. doi:10.3389/fmicb.2020.02050. PMC 7479814. PMID 32983036.
- Ho, Jonathan; Adeolu, Mobolaji; Khadka, Bijendra; Gupta, Radhey S. (October 2016). "Identification of distinctive molecular traits that are characteristic of the phylum "Deinococcus–Thermus" and distinguish its main constituent groups". Systematic and Applied Microbiology. 39 (7): 453–463. doi:10.1016/j.syapm.2016.07.003. ISSN 0723-2020. PMID 27506333.
- Hassan, F. M. Nazmul; Gupta, Radhey S. (2018-03-08). "Novel Sequence Features of DNA Repair Genes/Proteins from Deinococcus Species Implicated in Protection from Oxidatively Generated Damage". Genes. 9 (3): 149. doi:10.3390/genes9030149. ISSN 2073-4425. PMC 5867870. PMID 29518000.
- Inaba, Kenji; Ito, Koreaki (April 2008). "Structure and mechanisms of the DsbB–DsbA disulfide bond generation machine". Biochimica et Biophysica Acta (BBA) - Molecular Cell Research. 1783 (4): 520–529. doi:10.1016/j.bbamcr.2007.11.006. ISSN 0167-4889. PMID 18082634.
- J.P. Euzéby. "Deinococcus". List of Prokaryotic names with Standing in Nomenclature (LPSN). Retrieved 2022-07-20.
- Sayers; et al. "DeinococcusThermus". National Center for Biotechnology Information (NCBI) taxonomy database. Retrieved 2022-07-20.
- Wang W, Mao J, Zhang Z, Tang Q, Xie Y, Zhu J, Zhang L, Liu Z, Shi Y, Goodfellow M. Deinococcus wulumuqiensis sp. nov., and Deinococcus xibeiensis sp. nov., isolated from radiation-polluted soil. Int J Syst Evol Microbiol. 2010 Sep;60(Pt 9):2006-10
- Groot, Arjan de; Dulermo, Rémi; Ortet, Philippe; Blanchard, Laurence; Guérin, Philippe; Fernandez, Bernard; Vacherie, Benoit; Dossat, Carole; Jolivet, Edmond; Siguier, Patricia; Chandler, Michael; Barakat, Mohamed; Dedieu, Alain; Barbe, Valérie; Heulin, Thierry (2009-03-27). "Alliance of Proteomics and Genomics to Unravel the Specificities of Sahara Bacterium Deinococcus deserti". PLOS Genetics. 5 (3): e1000434. doi:10.1371/journal.pgen.1000434. ISSN 1553-7404. PMC 2669436. PMID 19370165.
- "The LTP_01_2022". The All-Species Living Tree Project. Retrieved 20 June 2022.
- "LTP_all tree in newick format". The All-Species Living Tree Project. Retrieved 20 June 2022.
- "LTP_01_2022 Release Notes" (PDF). The All-Species Living Tree Project. Retrieved 20 June 2022.
- "GTDB release 07-RS207". Genome Taxonomy Database. Retrieved 20 June 2022.
- "bac120_r207.sp_labels". Genome Taxonomy Database. Retrieved 20 June 2022.
- "Taxon History". Genome Taxonomy Database. Retrieved 20 June 2022.
- Rainey FA, Ferreira M, Nobre MF, Ray K, Bagaley D, Earl AM, Battista JR, Gómez-Silva B, McKay CP, da Costa MS. Deinococcus peraridilitoris sp. nov., isolated from a coastal desert. Int J Syst Evol Microbiol. 2007 Jul;57(Pt 7):1408-12.
- Weon HY, Kim BY, Schumann P, Son JA, Jang J, Go SJ, Kwon SW. Deinococcus cellulosilyticus sp. nov., isolated from air. Int J Syst Evol Microbiol. 2007 Aug;57(Pt 8):1685-8.
- Lewis NF. Radio-resistant Micrococcus radiophilus sp. nov. isolated from irradiated Bombay duck (Harpodon nehereus). Curr. Sci. (India)1976, v. 42, no. 14, p. 504
- Shashidhar R, Bandekar JR. Deinococcus piscis sp. nov., a radiation-resistant bacterium isolated from a marine fish. Int J Syst Evol Microbiol. 2009 Nov;59(Pt 11):2714-7
- Kobatake, M., Tanabe, S., Hasegawa, S. Nouveau micrococcus radioresistant a pigment rouge, isole de feces de Lama glama, et son utilisation comme indicateur microbiologique de la radiosterilisation. C.R. Seances Soc. Biol. Fil. (1973)167, 1506–1510.
- Rainey FA, Ray K, Ferreira M, Gatz BZ, Nobre MF, Bagaley D, Rash BA, Park MJ, Earl AM, Shank NC, Small AM, Henk MC, Battista JR, Kämpfer P, da Costa MS. Extensive diversity of ionizing-radiation-resistant bacteria recovered from Sonoran Desert soil and description of nine new species of the genus Deinococcus obtained from a single soil sample. Appl Environ Microbiol. 2005 Sep;71(9):5225-35. Erratum in: Appl Environ Microbiol. 2005 Nov;71(11):7630.
- Asker D, Awad TS, Beppu T, Ueda K. Deinococcus misasensis and Deinococcus roseus, novel members of the genus Deinococcus, isolated from a radioactive site in Japan. Syst Appl Microbiol. 2008 Mar;31(1):43-9.
- Parte, A.C. "Deinococcus". LPSN.
- Asker D, Awad TS, Beppu T, Ueda K. Deinococcus aquiradiocola sp. nov., isolated from a radioactive site in Japan. Int J Syst Evol Microbiol. 2009 Jan;59(Pt 1):144-9.
- Callegan RP, Nobre MF, McTernan PM, Battista JR, Navarro-González R, McKay CP, da Costa MS, Rainey FA. Description of four novel psychrophilic, ionizing radiation-sensitive Deinococcus species from alpine environments. Int J Syst Evol Microbiol. 2008 May;58(Pt 5):1252-8.
- Ferreira AC, Nobre MF, Rainey FA, Silva MT, Wait R, Burghardt J, Chung AP, da Costa MS. Deinococcus geothermalis sp. nov. and Deinococcus murrayi sp. nov., two extremely radiation-resistant and slightly thermophilic species from hot springs. Int J Syst Bacteriol. 1997 Oct;47(4):939-47.
- Yang Y, Itoh T, Yokobori S, Shimada H, Itahashi S, Satoh K, Ohba H, Narumi I, Yamagishi A. Deinococcus aetherius sp. nov., isolated from the stratosphere. Int J Syst Evol Microbiol. 2010 Apr;60(Pt 4):776-9
- Yang Y, Itoh T, Yokobori S, Itahashi S, Shimada H, Satoh K, Ohba H, Narumi I, Yamagishi A. Deinococcus aerius sp. nov., isolated from the high atmosphere. Int J Syst Evol Microbiol. 2009 Aug;59(Pt 8):1862-6.
- Lai WA, Kämpfer P, Arun AB, Shen FT, Huber B, Rekha PD, Young CC. Deinococcus ficus sp. nov., isolated from the rhizosphere of Ficus religiosa L. Int J Syst Evol Microbiol. 2006 Apr;56(Pt 4):787-91
- Anderson, A W; H C Nordan, R F Cain, G Parrish, D Duggan (1956). "Studies on a radio-resistant micrococcus. I. Isolation, morphology, cultural characteristics, and resistance to gamma radiation". Food Technol. 10 (1): 575–577.
- Peng F, Zhang L, Luo X, Dai J, An H, Tang Y, Fang C. Deinococcus xinjiangensis sp. nov., isolated from desert soil. Int J Syst Evol Microbiol. 2009 Apr;59(Pt 4):709-13.
- Chen W, Wang B, Hong H, Yang H, Liu SJ. Deinococcus reticulitermitis sp. nov., isolated from a termite gut.Int J Syst Evol Microbiol. 2011 Feb 18
- Yuan M, Zhang W, Dai S, Wu J, Wang Y, Tao T, Chen M, Lin M. Deinococcus gobiensis sp. nov., an extremely radiation-resistant bacterium. Int J Syst Evol Microbiol. 2009 Jun;59(Pt 6):1513-7
- Kämpfer P, Lodders N, Huber B, Falsen E, Busse HJ. Deinococcus aquatilis sp. nov., isolated from water. Int J Syst Evol Microbiol. 2008 Dec;58(Pt 12):2803-6.
- Zhang YQ, Sun CH, Li WJ, Yu LY, Zhou JQ, Zhang YQ, Xu LH, Jiang CL. Deinococcus yunweiensis sp. nov., a gamma- and UV-radiation-resistant bacterium from China. Int J Syst Evol Microbiol. 2007 Feb;57(Pt 2):370-5.
- Yoo SH, Weon HY, Kim SJ, Kim YS, Kim BY, Kwon SW. Deinococcus aerolatus sp. nov. and Deinococcus aerophilus sp. nov., isolated from air samples. Int J Syst Evol Microbiol. 2010 May;60(Pt 5):1191-5.
- Davis, N.S., Silverman, G.J., Mausurosky, E.B. Radiation-resistant, pigmented coccus isolated from haddock tissue. J. Bacteriol. 1963;86, 294–298.
- Hirsch P, Gallikowski CA, Siebert J, Peissl K, Kroppenstedt R, Schumann P, Stackebrandt E, Anderson R. Deinococcus frigens sp. nov., Deinococcus saxicola sp. nov., and Deinococcus marmoris sp. nov., low temperature and draught-tolerating, UV-resistant bacteria from continental Antarctica. Syst Appl Microbiol. 2004 Nov;27(6):636-45.
- de Groot A, Chapon V, Servant P, Christen R, Saux MF, Sommer S, Heulin T. Deinococcus deserti sp. nov., a gamma-radiation-tolerant bacterium isolated from the Sahara Desert. Int J Syst Evol Microbiol. 2005 Nov;55(Pt 6):2441-6.
- Suresh K, Reddy GS, Sengupta S, Shivaji S. Deinococcus indicus sp. nov., an arsenic-resistant bacterium from an aquifer in West Bengal, India. Int J Syst Evol Microbiol. 2004 Mar;54(Pt 2):457-61.
- Im WT, Jung HM, Ten LN, Kim MK, Bora N, Goodfellow M, Lim S, Jung J, Lee ST. Deinococcus aquaticus sp. nov., isolated from fresh water, and Deinococcus caeni sp. nov., isolated from activated sludge. Int J Syst Evol Microbiol. 2008 Oct;58(Pt 10):2348-53.
- Asker D, Awad TS, McLandsborough L, Beppu T, Ueda K. Deinococcus depolymerans sp. nov., a gamma- and UV-radiation-resistant bacterium, isolated from a naturally radioactive site. Int J Syst Evol Microbiol. 2011 Jun;61(Pt 6):1448-53
- Oyaizu H, Stackebrandt E, Schleifer KH, Ludwig W, Pohla H, Ito H, Hirata A, Oyaizu Y, Komagata K. A radiation-resistant rod-shaped bacterium, Deinobacter grandis gen. nov., sp. nov., with peptidoglycan containing ornithine. Int. J. Syst. Bacteriol., 1987, 37, 62-67.
- Rainey FA, Nobre MF, Schumann P, Stackebrandt E, Da Costa MS. Phylogenetic diversity of the deinococci as determined by 16S ribosomal DNA sequence comparison. Int. J. Syst. Bacteriol., 1997, 47, 510-514
- Srinivasan S, Kim MK, Lim S, Joe M, Lee M. Deinococcus daejeonensis sp. nov., isolated from sludge in a sewage disposal plant. Int J Syst Evol Microbiol. 2011 Jul 15