Esfenvalerate
Esfenvalerate is a synthetic pyrethroid insecticide marketed under the brand Asana.[2] It is the (S)-enantiomer of fenvalerate.[3]
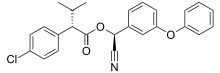 | |
| Names | |
|---|---|
| Preferred IUPAC name
(S)-Cyano(3-phenoxyphenyl)methyl (2S)-2-(4-chlorophenyl)-3-methylbutanoate | |
| Other names
Asana (S)-Fenvalerate | |
| Identifiers | |
3D model (JSmol) |
|
| 4275674 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.118.804 |
| EC Number |
|
| KEGG | |
PubChem CID |
|
| UNII | |
| UN number | 3349 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C25H22ClNO3 | |
| Molar mass | 419.91 g·mol−1 |
| Density | 1.211 g/cm3 |
| Melting point | 60 °C (140 °F; 333 K) |
| log P | 6.22 |
| Vapor pressure | 0 mmHg at 25 °C |
| Hazards | |
| GHS labelling: | |
    | |
| Danger | |
| H301, H313, H316, H317, H320, H330, H335, H370, H373, H410 | |
| P260, P261, P264, P270, P271, P272, P273, P280, P284, P301+P310, P302+P352, P304+P340, P305+P351+P338, P307+P311, P310, P312, P314, P320, P321, P330, P332+P313, P333+P313, P337+P313, P363, P391, P403+P233, P405, P501 | |
| Flash point | 256 °C (493 °F; 529 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
In the United States, a limit of .05 ppm of the chemical's residue is permissible in food.[4]
References
- Kelly, Kevin. "Environmental Fate of Esfenvalerate". California Environmental Protection Agency. Retrieved January 10, 2013.
- Fishel, Frederick M. (2012). "Pesticide Toxicity Profile: Synthetic Pyrethroid Pesticides". University of Florida. Retrieved January 10, 2013.
- "Esfenvalerate". EXTONET (Extension Toxicology Network). Cooperative Extension Offices of Cornell University, Michigan State University, Oregon State University, and University of California at Davis. May 1994. Retrieved January 10, 2013.
- The Code of Federal Regulations of the United States of America. U.S. Government Printing Office. 2006. pp. 445–446.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.