Fenoprop
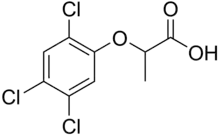
Fenoprop, also called 2,4,5-TP, is the organic compound 2-(2,4,5-trichlorophenoxy)propionic acid.[2] It is a phenoxy herbicide and a plant growth regulator, an analog of 2,4,5-T in which the latter's acetic acid sidechain is replaced with a propionate group (with an extra CH3). The addition of this extra methyl group creates a chiral centre in the molecule and useful biological activity is found only in the (2R)-isomer.[3] The compound's mechanism of action is to mimic the auxin growth hormone indoleacetic acid (IAA).[4] When sprayed on plants it induces rapid, uncontrolled growth. As with 2,4,5-T, fenoprop is toxic to shrubs and trees.
 | |
| Names | |
|---|---|
| Preferred IUPAC name
rac-(2R)-2-(2,4,5-trichlorophenoxy)propanoic acid | |
| Other names
2-(2,4,5-Trichlorophenoxy)propionic acid Silvex | |
| Identifiers | |
3D model (JSmol) |
|
| Abbreviations | 2,4,5-TP |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.002.066 |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties[1] | |
| C9H7Cl3O3 | |
| Molar mass | 269.51 |
| Appearance | White powder |
| Density | 1.21 g/cm3 at 20 °C |
| Melting point | 180 °C (356 °F; 453 K) |
| log P | 3.8 (20 °C) |
| Acidity (pKa) | 2.84 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
The name Silvex was used in the USA but it has been banned from use there since 1985. According to the Environmental Protection Agency its greatest use was as a postemergence herbicide for control of woody plants, and broadleaf herbaceous weeds in rice and bluegrass turf, in sugarcane, in rangeland improvement programs and on lawns.[5] Fenoprop and some of its esters were in use from 1945 but are now obsolete.[1]
References
- Pesticide Properties Database. "Fenoprop". University of Hertfordshire. Retrieved 2021-03-11.
- "Compendium of Pesticide Common Names". alanwood.net.
- Wendeborn, S.; Smits, H. (31 December 2012). "Synthetic Auxins". In Erick M. Carreira; Hisashi Yamamoto (eds.). Comprehensive Chirality. ISBN 9780080951683.
- Grossmann, K. (2010). "Auxin herbicides: current status of mechanism and mode of action". Pest Management Science. 66 (2): 2033–2043. doi:10.1002/ps.1860. PMID 19823992.
- US Environmental Protection Agency. "Consumer Factsheet on: 2,4,5-TP (SILVEX)" (PDF). Retrieved 2021-03-11.