Quinclorac
Quinclorac is a selective herbicide used primarily to control weeds in rice crops,[1] but is also used on other agricultural crops and is found in some household herbicides for lawn use. Most lawn maintenance companies use the product for the control of annual grass weeds like crabgrass.
 | |
| Names | |
|---|---|
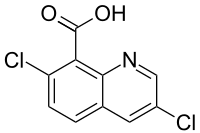
| Preferred IUPAC name
3,7-Dichloroquinoline-8-carboxylic acid | |
| Other names
Quinchlorac | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.100.457 |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C10H5Cl2NO2 | |
| Molar mass | 242.06 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Quinclorac is considered a synthetic auxin.[1][2] Heap considers it to also have a cellulose herbicide action[3] although some studies show quinclorac to have no cellulose action.[4]
It is not approved to use in the European Union,[5] and has a high class 3 threshold of toxilogical concern.[6]
Resistance
Resistance to quinclorac is of concern in soybean cultivation.[7] In rice, Guo et al. 2019 find that the natural Graminaceous resistance is produced by the cytochrome P450 CYP81A6.[8]
References
- Grossmann, Klaus (1998). "Quinclorac belongs to a new class of highly selective auxin herbicides". Weed Science. 46 (6): 707–716. doi:10.1017/S004317450008975X. S2CID 89297943.
- Grossmann, Klaus; Kwiatkowski, Jacek (2000). "The Mechanism of Quinclorac Selectivity in Grasses". Pesticide Biochemistry and Physiology. Elsevier. 66 (2): 83–91. doi:10.1006/pest.1999.2461. ISSN 0048-3575. S2CID 84092985.
- Heap, Ian. "List of Herbicide Resistant Weeds by Herbicide Mode of Action (L/26)". International Survey of Herbicide Resistant Weeds. Herbicide Resistance Action Committee. Retrieved 2021-03-14.
-
- Alonso-Simón, Ana; García-Angulo, Penélope; Mélida, Hugo; Encina, Antonio; Álvarez, Jesús M.; Acebes, José L. (2011). "The use of FTIR spectroscopy to monitor modifications in plant cell wall architecture caused by cellulose biosynthesis inhibitors". Mini Review. Plant Signaling & Behavior. 6 (8): 1104–1110. doi:10.4161/psb.6.8.15793. PMC 3260703. PMID 21791979.
- This review cites this research.
- Tresch, Stefan; Grossmann, Klaus (2003). "Quinclorac does not inhibit cellulose (cell wall) biosynthesis in sensitive barnyard grass and maize roots". Pesticide Biochemistry and Physiology. Elsevier. 75 (3): 73–78. doi:10.1016/s0048-3575(03)00013-0. ISSN 0048-3575. S2CID 84212641.
- Tresch, Stefan; Grossmann, Klaus (2003). "Erratum to "Quinclorac does not inhibit cellulose (cell wall) biosynthesis in sensitive barnyard grass and maize roots"". Pesticide Biochemistry and Physiology. Elsevier. 76 (2): 70–71. doi:10.1016/s0048-3575(03)00064-6. ISSN 0048-3575. S2CID 84794877.
- "Quinclorac".
- "Quinclorac (Ref: BAS 514H)".
- "Barnyard Management in Soybeans Fact Sheet". I Will Take Action. Soybean Checkoff. 2021.
{{cite web}}: Missing or empty|url=(help) - Gaines, Todd A.; Duke, Stephen O.; Morran, Sarah; Rigon, Carlos A.G.; Tranel, Patrick J.; Küpper, Anita; Dayan, Franck E. (2020). "Mechanisms of Evolved Herbicide Resistance". Journal of Biological Chemistry. Elsevier BV. 295 (30): 10307–10330. doi:10.1074/jbc.rev120.013572. PMC 7383398. PMID 32430396.