Gadolinium(III) oxalate
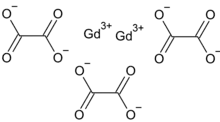
Gadolinium oxalate is the oxalate of gadolinium, with the chemical formula Gd2(C2O4)3. Its hydrate can be prepared by the reaction of gadolinium nitrate and oxalic acid.[1]
 | |
| Identifiers | |
|---|---|
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.011.606 |
| EC Number |
|
PubChem CID |
|
| |
| |
| Properties | |
| Gd2(C2O4)3 | |
| Appearance | colorless crystals |
| insoluble | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Properties
The decahydrate of gadolinium oxalate thermally decomposes to obtain the anhydrous form, which can then be heated to produce gadolinium oxide.[2] Gadolinium oxalate reacts with hydrochloric acid to produce Gd(C2O4)Cl.[3] It also reacts with sodium hydroxide under hydrothermal conditions to produce gadolinium hydroxide.[1]
References
- Yidong Yin, Guangyan Hong (2006-11-03). "Synthesis and characterization of Gd(OH)3 nanobundles". Journal of Nanoparticle Research. 8 (5): 755–760. Bibcode:2006JNR.....8..755Y. doi:10.1007/s11051-005-9044-7. ISSN 1388-0764. S2CID 98381833. Retrieved 2020-10-11.
- Wendlandt, W. W. (1959). "Thermal Decomposition of Rare Earth Metal Oxalates". Analytical Chemistry. 31 (3): 408–410. doi:10.1021/ac60147a024. ISSN 0003-2700.
- Moebius, R.; Matthes, F. (1964). "The exchange of oxalate ions for chloride ions of the oxalate hydrates of the rare earths and yttrium". Zeitschrift für Chemie. 4 (6): 234–235. ISSN 0044-2402.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.