Glioma
A glioma is a type of tumor that starts in the glial cells of the brain or the spine.[1] Gliomas comprise about 30 percent of all brain tumors and central nervous system tumours, and 80 percent of all malignant brain tumours.[2]
| Glioma | |
|---|---|
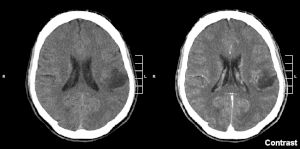 | |
| Glioma in the left parietal lobe (brain CT scan), WHO grade 2 | |
| Specialty | Oncology, Neurology |
Signs and symptoms
Symptoms of gliomas depend on which part of the central nervous system is affected. A brain glioma can cause headaches, vomiting, seizures, and cranial nerve disorders as a result of increased intracranial pressure. A glioma of the optic nerve can cause vision loss. Spinal cord gliomas can cause pain, weakness, or numbness in the extremities. Gliomas do not usually metastasize by the bloodstream, but they can spread via the cerebrospinal fluid and cause "drop metastases" to the spinal cord. Complex visual hallucinations have been described as a symptom of low-grade glioma.[3]
A child who has a subacute disorder of the central nervous system that produces cranial nerve abnormalities (especially of cranial nerve VII and the lower bulbar nerves), long-tract signs, unsteady gait secondary to spasticity, and some behavioral changes is most likely to have a pontine glioma.[4]
Causes
Hereditary disorders
The exact causes of gliomas are not known. Hereditary disorders such as neurofibromatoses (type 1 and type 2) and tuberous sclerosis complex are known to predispose to their development.[5] Different oncogenes can cooperate in the development of gliomas.[6]
Diet
Some studies of diet and vitamin supplementation appear to point out that dietary N-nitroso compounds may affect the risk of both childhood and adult brain tumors. Researchers have observed in some studies that brain tumor patients (or their mothers) have generally consumed more cured foods (also known as Curing) than control groups. Recently, Drs. Lee, Wrensch and others found that adults with glioma were more likely to consume diets high in cured foods and low in vitamin C-rich fruits and vegetables, and to consume diets high in nitrites and low in vitamin C. The effect was more pronounced in men than women. However, the pattern of increased risk with increased consumption of cured foods, and decreased risk with greater consumption of fruits, vegetables, and antioxidant vitamins is compatible with other cancer studies that show increased consumption of vegetables and possibly of fruits is associated with decreased cancer risk.[7]
Radiation
The best-known risk factor is exposure to ionizing radiation, and CT scan radiation is an important cause.[8][9] The dose-response for the relationship between low-dose ionising radiation and glioma risk is a risk increase of 55% per 100 milligray of radiation.[8] A link between gliomas and electromagnetic radiation from cell phones has not been conclusively proven.[10] It was considered possible,[11][12] though several large studies have found no conclusive evidence, as summarized by the NIH's National Cancer Institute review of the topic[13] and its numerous citations,[14] and the FCC.[15] However, further research is still being pursued to obtain more robust evidence and verify that there is no relationship (the NIH's National Institute of Environmental Health Sciences most recent press release discussed an ongoing study[16] showing mildly positive results,[17] although it appears there may have been issues with the control group dying prematurely[18]).
Infection with cytomegalovirus
Some studies have reported that glioblastomas are infected with cytomegalovirus, with suggestions that this may speed the development of tumors.[19][20][21] However, this is a controversial opinion, with recent in-depth studies failing to find an association between viral infection and glioma growth.[22] There is also evidence that previous studies may have been impacted by false-positive antibody staining artifacts.[23]
Other causes
Though some studies have shown that farmers have higher rates of gliomas compared to the general population, exposure to farm animals or manure is not associated with glioma.[24][25] Later studies have not found an association between farming and gliomas; similar conflicting data concerns teachers and glioma. More consistent data show that architects, surveyors, retail workers, butchers, and engineers have higher rates of gliomas.[26] Most studies have found that pesticide exposure is probably not a cause of glioma, though a minority of studies have found an association.[26][27][28][29]
Inherited polymorphisms of the DNA repair genes
Germ-line (inherited) polymorphisms of the DNA repair genes ERCC1, ERCC2 (XPD) and XRCC1 increase the risk of glioma.[30] This indicates that altered or deficient repair of DNA damage contributes to the formation of gliomas. DNA damages are a likely major primary cause of progression to cancer in general.[31] Excess DNA damages can give rise to mutations through translesion synthesis. Furthermore, incomplete DNA repair can give rise to epigenetic alterations or epimutations.[32][33] Such mutations and epimutations may provide a cell with a proliferative advantage which can then, by a process of natural selection, lead to progression to cancer.[31]
Epigenetic repression of DNA repair genes is often found in progression to sporadic glioblastoma. For instance, methylation of the DNA repair gene MGMT promoter was observed in 51% to 66% of glioblastoma specimens.[34][35] In addition, in some glioblastomas, the MGMT protein is deficient due to another type of epigenetic alteration. MGMT protein expression may also be reduced due to increased levels of a microRNA that inhibits the ability of the MGMT messenger RNA to produce the MGMT protein.[35] Zhang et al.[36] found, in the glioblastomas without methylated MGMT promoters, that the level of microRNA miR-181d is inversely correlated with protein expression of MGMT and that the direct target of miR-181d is the MGMT mRNA 3'UTR (the three prime untranslated region of MGMT messenger RNA).[37]
Epigenetic reductions in expression of another DNA repair protein, ERCC1, were found in an assortment of 32 gliomas.[38] For 17 of the 32 (53%) of the gliomas tested, ERCC1 protein expression was reduced or absent. In the case of 12 gliomas (37.5%) this reduction was due to methylation of the ERCC1 promoter. For the other 5 gliomas with reduced ERCC1 protein expression, the reduction could have been due to epigenetic alterations in microRNAs that affect ERCC1 expression.[39]
When expression of DNA repair genes is reduced, DNA damages accumulate in cells at a higher than normal level, and such excess damages cause increased frequencies of mutation.[40][41][42] Mutations in gliomas frequently occur in either isocitrate dehydrogenase (IDH) 1 or 2 genes.[43] One of these mutations (mostly in IDH1) occurs in about 80% of low grade gliomas and secondary high-grade gliomas.[44] Wang et al.[45] pointed out that IDH1 and IDH2 mutant cells produce an excess metabolic intermediate, 2-hydroxyglutarate, which binds to catalytic sites in key enzymes that are important in altering histone and DNA promoter methylation. Thus, mutations in IDH1 and IDH2 generate a "DNA CpG island methylator phenotype or CIMP"[46][47] that causes promoter hypermethylation and concomitant silencing of tumor suppressor genes such as DNA repair genes MGMT and ERCC1. On the other hand, Cohen et al.[44] and Molenaar et al.[43] pointed out that mutations in IDH1 or IDH2 can cause increased oxidative stress. Increased oxidative damage to DNA could be mutagenic. This is supported by an increased number of DNA double-strand breaks in IDH1-mutated glioma cells.[48] Thus, IDH1 or IDH2 mutations act as driver mutations in glioma carcinogenesis, though it is not clear by which role they are primarily acting. A study, involving 51 patients with brain gliomas who had two or more biopsies over time, showed that mutation in the IDH1 gene occurred prior to the occurrence of a p53 mutation or a 1p/19q loss of heterozygosity, indicating that an IDH1 mutation is an early driver mutation.[49]
Pathophysiology
High-grade gliomas are highly vascular tumors and have a tendency to infiltrate diffusely.[50] They have extensive areas of necrosis and hypoxia. Often, tumor growth causes a breakdown of the blood–brain barrier in the vicinity of the tumor. As a rule, high-grade gliomas almost always grow back even after complete surgical excision, so are commonly called recurrent cancer of the brain.[51]
Conversely, low-grade gliomas grow slowly, often over many years, and can be followed without treatment unless they grow and cause symptoms.
Several acquired (not inherited) genetic mutations have been found in gliomas. Tumor suppressor protein 53 (p53) is mutated early in the disease.[52] p53 is the "guardian of the genome", which, during DNA and cell duplication, makes sure the DNA is copied correctly and destroys the cell (apoptosis) if the DNA is mutated and cannot be fixed. When p53 itself is mutated, other mutations can survive. Phosphatase and tensin homolog (PTEN), another tumor suppressor gene, is itself lost or mutated. Epidermal growth factor receptor, a growth factor that normally stimulates cells to divide, is amplified and stimulates cells to divide too much. Together, these mutations lead to cells dividing uncontrollably, a hallmark of cancer. In 2009, mutations in IDH1 and IDH2 were found to be part of the mechanism and associated with a less favorable prognosis.[53]
Diagnosis
Classification
Gliomas are classified by cell type, by grade, and by location.
By type of cell
Gliomas are named according to the specific type of cell with which they share histological features, but not necessarily from which they originate. The main types of glioma are:[54]
- Ependymomas: ependymal cells
- Astrocytomas: astrocytes (glioblastoma multiforme is a malignant astrocytoma and the most common primary brain tumor among adults).
- Oligodendrogliomas: oligodendrocytes
- Brainstem glioma: develop in the brain stem
- Optic nerve glioma: develop in or around the optic nerve
- Mixed gliomas, such as oligoastrocytomas, contain cells from different types of glia
By grade
Gliomas are further categorised according to their grade, which is determined by pathologic evaluation of the tumor. The neuropathological evaluation and diagnostics of brain tumor specimens is performed according to WHO Classification of Tumours of the Central Nervous System.[55][56]
- Biologically benign gliomas [WHO grade I] are comparatively low risk and can be removed surgically depending on their location[50]
- Low-grade gliomas [WHO grade II] are well-differentiated (not anaplastic); these tend to exhibit benign tendencies and portend a better prognosis for the patient. However, they have a uniform rate of recurrence and increase in grade over time so should be classified as malignant.
- High-grade [WHO grades III–IV] gliomas are undifferentiated or anaplastic; these are malignant and carry a worse prognosis. Despite being classified as a high-grade glioma, infant-type hemispheric gliomas have relatively good clinical outcomes, yet they endure significant deficits, making them good candidates for therapy de-escalation and trials of molecular targeted therapy.[57]
Of numerous grading systems in use, the most common is the World Health Organization (WHO) grading system for astrocytoma, under which tumors are graded from I (least advanced disease—best prognosis) to IV (most advanced disease—worst prognosis).
By location
Gliomas can be classified according to whether they are above or below a membrane in the brain called the tentorium.[58] The tentorium separates the cerebrum (above) from the cerebellum (below).
- The supratentorial is above the tentorium, in the cerebrum, and mostly found in adults (70%).[59]
- The infratentorial is below the tentorium, in the cerebellum, and mostly found in children (70%).[59]
- The pontine tumors are located in the pons of the brainstem. The brainstem has three parts (pons, midbrain, and medulla); the pons controls critical functions such as breathing, making surgery on these extremely dangerous.[60]
Treatment
Treatment for brain gliomas depends on the location, the cell type, and the grade of malignancy. Current treatment options include surgical removal, radiation (radiation therapy), and chemotherapy. In some cases, tumour treating fields (alternating electric field therapy), a recently developed technology, may be used.[61] Often, treatment is a combined approach, using surgery, radiation therapy, and chemotherapy. For many, treatment consists of just surgery, or even "watchful waiting" (waiting to see when an intervention is justified due to tumour progression). Doctors carefully balance the specifics of the patient's tumour and the downsides of intervention, since there can be significant side effects from medical intervention.
Radiation and chemotherapy remain the mainstays of treatment beyond surgery. Radiation therapy is delivered in the form of external beam radiation or the stereotactic approach using radiosurgery. Temozolomide is a common chemotherapy drug which can be administered easily in an outpatient setting and is able to cross the blood–brain barrier effectively.
There are a wide variety of novel treatments currently being tested in clinical trials, ranging from IDH inhibitors like Ivosidenib, to the recently approved Dendritic cell-based cancer vaccine approach.[62] Treatment using immunotherapy is another promising research path that may help treat glioma in the near future.[63][64] Experimental therapies like oncolytic viruses have shown potential therapeutic benefits in clinical trials (but have not been approved for use in non-experimental settings).[65]
Refractory disease
For recurrent high-grade glioblastoma, recent studies have taken advantage of angiogenic blockers such as bevacizumab in combination with conventional chemotherapy, with encouraging results.[66]
Relative effectiveness
A 2017 meta-analysis compared surgical resection versus biopsy as the initial surgical management option for a person with a low-grade glioma.[67] Results show the evidence is insufficient to make a reliable decision.[67] The relative effectiveness of surgical resection compared to biopsy for people with malignant glioma (high-grade) is unknown.[68]
For high-grade gliomas, a 2003 meta-analysis compared radiotherapy with radiotherapy and chemotherapy. It showed a small but clear improvement from using chemotherapy with radiotherapy.[69] A 2019 meta-analysis suggested that for people with less aggressive gliomas, radiotherapy may increase the risk of long term neurocognitive side effects.[70] Whilst, evidence is uncertain on whether there are long term neurocognitive side effects associated with chemoradiotherapy.[70]
Temozolomide is effective for treating Glioblastoma Multiforme (GBM) compared to radiotherapy alone.[62] A 2013 meta-analysis showed that Temozolomide prolongs survival and delays progression, but is associated with an increase in side effects such as blood complications, fatigue, and infection.[62] For people with recurrent GBM, when comparing temozolomide with chemotherapy, there may be an improvement in the time-to-progression and the person's quality of life, but no improvement in overall survival, with temozolomide treatment.[62] Evidence suggests that for people with recurrent high-grade gliomas who have not had chemotherapy before, there are similar survival and time-to-progression outcomes between treatment with temozolomide or the chemotherapy multidrug known as PCV (procarvazine, lomustine and vincristine).[71]
A mutational analysis of 23 initial low-grade gliomas and recurrent tumors from the same patients has challenged the benefits and usage of Temozolomide. The study showed that when lower-grade brain tumors of patients are removed and patients are further treated with Temozolomide, 6 out of 10 times the recurrent tumors were more aggressive and acquired alternative and more mutations.[72] As one of the last authors, Costello, stated "They had a 20- to 50-fold increase in the number of mutations. A patient who received surgery alone who might have had 50 mutations in the initial tumor and 60 in the recurrence. But patients who received TMZ might have 2,000 mutations in the recurrence."[73] Further, new mutations were verified to carry known signatures of Temozolomide induced mutations. The research suggests that Temozolomide for the treatment of certain brain tumors should be thoroughly thought. Unjudicious usage of Temozolomide might lower the prognosis of the patients further, or increase their burden. Further understanding of the mechanisms of Temozolomide induced mutations and novel combination approaches could be promising.
Prognosis
Prognosis of gliomas is given in relation to what grade (as scored by the World Health Organization system) of tumour the patient presents with. Typically, any tumour presenting as above WHO grade I (i.e. a malignant tumour as opposed to a benign tumour) will have a prognosis resulting in eventual death, varying from years (WHO grade II/III) to months (WHO grade IV).[50][74] Prognosis can also be given based on cellular subtype, which may also impact prognosis.
Low grade
For low-grade tumors, the prognosis is somewhat more optimistic. Patients diagnosed with a low-grade glioma are 17 times as likely to die as matched patients in the general population.[75] The age-standardized 10-year relative survival rate was 47%.[75] One study reported that low-grade oligodendroglioma patients have a median survival of 11.6 years;[76] another reported a median survival of 16.7 years.[77] Unfortunately, approximately 70% of low-grade (WHO grade-II) will progress to high-grade tumours within 5–10 years[50] Grade II gliomas, despite often being labeled as benign, are considered a uniformly fatal illness.[78]
High grade
This group comprises anaplastic astrocytomas and glioblastoma multiforme. Whereas the median overall survival of anaplastic (WHO grade III) gliomas is approximately 3 years, glioblastoma multiforme has a poor median overall survival of c. 15 months.[79]
Postoperative conventional daily radiotherapy improves survival for adults with good functional well‐being and high grade glioma compared to no postoperative radiotherapy. Hypofractionated radiation therapy has similar efficacy for survival as compared to conventional radiotherapy, particularly for individuals aged 60 and older with glioblastoma.[80]
Diffuse midline glioma
Diffuse midline glioma (DMG), previously known as diffuse intrinsic pontine glioma (DIPG), primarily affects children, usually between the ages of 5 and 7.[81] The median survival time with DIPG is under 12 months.[82] Surgery to attempt tumour removal is usually not possible or advisable for pontine gliomas. By their very nature, these tumours invade diffusely throughout the brain stem, growing between normal nerve cells. Aggressive surgery would cause severe damage to neural structures vital for arm and leg movement, eye movement, swallowing, breathing, and even consciousness.[83][84] Trials of drug candidates have been unsuccessful.[85] The disease is primarily treated with radiation therapy alone.
IDH1 and IDH2-mutated glioma
Patients with glioma carrying mutations in either IDH1 or IDH2 have a relatively favorable survival, compared with patients with glioma with wild-type IDH1/2 genes. In WHO grade III glioma, IDH1/2-mutated glioma have a median prognosis of ~3.5 years, whereas IDH1/2 wild-type glioma perform poor with a median overall survival of c. 1.5 years.[43] In glioblastoma, the difference is larger. There, IDH1/2 wild-type glioblastoma have a median overall survival of 1 year, whereas IDH1/2-mutated glioblastoma have a median overall survival of more than 3 years.[86]
References
- Mamelak AN, Jacoby DB (March 2007). "Targeted delivery of antitumoral therapy to glioma and other malignancies with synthetic chlorotoxin (TM-601)". Expert Opinion on Drug Delivery. 4 (2): 175–86. doi:10.1517/17425247.4.2.175. PMID 17335414. S2CID 20356267.
- Goodenberger ML, Jenkins RB (December 2012). "Genetics of adult glioma". Cancer Genetics. 205 (12): 613–21. doi:10.1016/j.cancergen.2012.10.009. PMID 23238284.
- Lim A, Weir P, O'Brien TJ, Kaye AH (January 2011). "Complex visual hallucinations as a presentation of temporal low-grade glioma". Journal of Clinical Neuroscience. 18 (1): 157–9. doi:10.1016/j.jocn.2010.07.112. PMID 20965734. S2CID 34392868.
- PRETEST pediatrics p. 224
- Reuss D, von Deimling A (2009). "Hereditary tumor syndromes and gliomas". Recent Results in Cancer Research. Fortschritte der Krebsforschung. Progres dans les Recherches Sur le Cancer. Recent Results in Cancer Research. 171: 83–102. doi:10.1007/978-3-540-31206-2_5. ISBN 978-3-540-31205-5. PMID 19322539.
- Radner H, el-Shabrawi Y, Eibl RH, Brüstle O, Kenner L, Kleihues P, Wiestler OD (1993). "Tumor induction by ras and myc oncogenes in fetal and neonatal brain: modulating effects of developmental stage and retroviral dose". Acta Neuropathologica. 86 (5): 456–65. doi:10.1007/bf00228580. PMID 8310796. S2CID 2972931.
- "Archived copy" (PDF). Archived from the original (PDF) on 23 November 2018. Retrieved 7 November 2016.
{{cite web}}: CS1 maint: archived copy as title (link) - Smoll NR, Brady Z, Scurrah KJ, Lee C, Berrington de González A, Mathews JD. Computed tomography scan radiation and brain cancer incidence. Neuro-Oncology. 2023 Jan 14;https://doi.org/10.1093/neuonc/noad012
- Smoll NR, Brady Z, Scurrah K, Mathews JD. Exposure to ionizing radiation and brain cancer incidence: The Life Span Study cohort. Cancer Epidemiology. 2016 Jun;42:60–5.
- Söderqvist F, Carlberg M, Hansson Mild K, Hardell L (December 2011). "Childhood brain tumour risk and its association with wireless phones: a commentary". Environmental Health. 10 (106): 106. doi:10.1186/1476-069X-10-106. PMC 3278351. PMID 22182218.
- Morgan LL, Kesari S, Davis DL (2014). "Why children absorb more microwave radiation than adults: The consequences". Journal of Microscopy and Ultrastructure. 4 (2): 197–204. doi:10.1016/j.jmau.2014.06.005.
- "IARC classifies radiofrequency electromagnetic fields as possibly carcinogenic to humans" (PDF) (Press release). IARC. 31 May 2011.
- "Cell Phones and Cancer Risk". National Cancer Institute. Retrieved 29 May 2016.
- "Cell Phones and Cancer Risk (References)". National Cancer Institute. Retrieved 29 May 2016.
- "Wireless Devices and Health Concerns". Federal Communications Commission (FCC). 26 May 2011. Retrieved 29 May 2016.
- "Media Telebriefing: NTP Cell Phone Radiofrequency Radiation Study: Partial Release of Findings". niehs.nih.gov (Press release). Retrieved 29 May 2016.
- Wyde M, Cesta M, Blystone C, et al. (1 January 2018). "Report of Partial findings from the National Toxicology Program Carcinogenesis Studies of Cell Phone Radiofrequency Radiation in Hsd: Sprague Dawley SD rats (Whole Body Exposure)". bioRxiv 10.1101/055699.
- Storrs C (27 May 2016). "Cell phone radiation increases cancers in rats, but should we worry?". CNN. Retrieved 29 May 2016.
- Michaelis M, Baumgarten P, Mittelbronn M, Driever PH, Doerr HW, Cinatl J (February 2011). "Oncomodulation by human cytomegalovirus: novel clinical findings open new roads". Medical Microbiology and Immunology. 200 (1): 1–5. doi:10.1007/s00430-010-0177-7. PMID 20967552. S2CID 22444291.
- Barami K (July 2010). "Oncomodulatory mechanisms of human cytomegalovirus in gliomas". Journal of Clinical Neuroscience. 17 (7): 819–23. doi:10.1016/j.jocn.2009.10.040. PMID 20427188. S2CID 6952978.
- Dziurzynski K, Chang SM, Heimberger AB, Kalejta RF, McGregor Dallas SR, Smit M, et al. (March 2012). HCMV and Gliomas Symposium. "Consensus on the role of human cytomegalovirus in glioblastoma". Neuro-Oncology. 14 (3): 246–55. doi:10.1093/neuonc/nor227. PMC 3280809. PMID 22319219.
- Strong, MJ; Blanchard E, 4th; Lin, Z; Morris, CA; Baddoo, M; Taylor, CM; Ware, ML; Flemington, EK (11 July 2016). "A comprehensive next generation sequencing-based virome assessment in brain tissue suggests no major virus — tumor association". Acta Neuropathologica Communications. 4 (1): 71. doi:10.1186/s40478-016-0338-z. PMC 4940872. PMID 27402152.
- Holdhoff, M; Guner, G; Rodriguez, FJ; Hicks, JL; Zheng, Q; Forman, MS; Ye, X; Grossman, SA; Meeker, AK; Heaphy, CM; Eberhart, CG; De Marzo, AM; Arav-Boger, R (15 June 2017). "Absence of Cytomegalovirus in Glioblastoma and Other High-grade Gliomas by Real-time PCR, Immunohistochemistry, and In Situ Hybridization". Clinical Cancer Research. 23 (12): 3150–3157. doi:10.1158/1078-0432.CCR-16-1490. PMC 5474132. PMID 28034905.
- Efird JT, Davies SW, O'Neal WT, Anderson EJ (2014). "Animal viruses, bacteria, and cancer: a brief commentary". Frontiers in Public Health. 2: 14. doi:10.3389/fpubh.2014.00014. PMC 3923154. PMID 24592380.
- Ruder AM, Carreón T, Butler MA, Calvert GM, Davis-King KE, Waters MA, et al. (June 2009). "Exposure to farm crops, livestock, and farm tasks and risk of glioma: the Upper Midwest Health Study". American Journal of Epidemiology. 169 (12): 1479–91. doi:10.1093/aje/kwp075. PMID 19403843.
- Ostrom QT, Bauchet L, Davis FG, Deltour I, Fisher JL, Langer CE, et al. (July 2014). "The epidemiology of glioma in adults: a "state of the science" review". Neuro-Oncology. 16 (7): 896–913. doi:10.1093/neuonc/nou087. PMC 4057143. PMID 24842956.
- "Women's Safety and Health Issues at Work: Job Area: Agriculture". National Institute for Occupational Safety and Health (NIOSH). Archived from the original on 22 August 2015. Retrieved 20 June 2015.
- Carreón T, Butler MA, Ruder AM, Waters MA, Davis-King KE, Calvert GM, et al. (May 2005). "Gliomas and farm pesticide exposure in women: the Upper Midwest Health Study". Environmental Health Perspectives. 113 (5): 546–51. doi:10.1289/ehp.7456. PMC 1257545. PMID 15866761.
- Yiin JH, Ruder AM, Stewart PA, Waters MA, Carreón T, Butler MA, et al. (June 2012). "The Upper Midwest Health Study: a case-control study of pesticide applicators and risk of glioma". Environmental Health. 11: 39. doi:10.1186/1476-069X-11-39. PMC 3406961. PMID 22691464.
- Adel Fahmideh M, Schwartzbaum J, Frumento P, Feychting M (June 2014). "Association between DNA repair gene polymorphisms and risk of glioma: a systematic review and meta-analysis". Neuro-Oncology. 16 (6): 807–14. doi:10.1093/neuonc/nou003. PMC 4022225. PMID 24500421.
- Bernstein, Carol; R., Anil; Nfonsam, Valentine; Bernstei, Harris (22 May 2013), Chen, Clark (ed.), "DNA Damage, DNA Repair and Cancer", New Research Directions in DNA Repair, InTech, doi:10.5772/53919, ISBN 978-953-51-1114-6, retrieved 3 July 2022
- Cuozzo C, Porcellini A, Angrisano T, Morano A, Lee B, Di Pardo A, et al. (July 2007). "DNA damage, homology-directed repair, and DNA methylation". PLOS Genetics. 3 (7): e110. doi:10.1371/journal.pgen.0030110. PMC 1913100. PMID 17616978.
- O'Hagan HM, Mohammad HP, Baylin SB (August 2008). "Double strand breaks can initiate gene silencing and SIRT1-dependent onset of DNA methylation in an exogenous promoter CpG island". PLOS Genetics. 4 (8): e1000155. doi:10.1371/journal.pgen.1000155. PMC 2491723. PMID 18704159.
- Skiriute D, Vaitkiene P, Saferis V, Asmoniene V, Skauminas K, Deltuva VP, Tamasauskas A (June 2012). "MGMT, GATA6, CD81, DR4, and CASP8 gene promoter methylation in glioblastoma". BMC Cancer. 12: 218. doi:10.1186/1471-2407-12-218. PMC 3404983. PMID 22672670.
- Spiegl-Kreinecker S, Pirker C, Filipits M, Lötsch D, Buchroithner J, Pichler J, et al. (January 2010). "O6-Methylguanine DNA methyltransferase protein expression in tumor cells predicts outcome of temozolomide therapy in glioblastoma patients". Neuro-Oncology. 12 (1): 28–36. doi:10.1093/neuonc/nop003. PMC 2940563. PMID 20150365.
- Zhang W, Zhang J, Hoadley K, Kushwaha D, Ramakrishnan V, Li S, et al. (June 2012). "miR-181d: a predictive glioblastoma biomarker that downregulates MGMT expression". Neuro-Oncology. 14 (6): 712–9. doi:10.1093/neuonc/nos089. PMC 3367855. PMID 22570426.
- Zhang, Wei; Zhang, Jing; Hoadley, Katherine; Kushwaha, Deepa; Ramakrishnan, Valya; Li, Shouwei; Kang, Chunsheng; You, Yongping; Jiang, Chuanlu; Song, Sonya Wei; Jiang, Tao (1 June 2012). "miR-181d: a predictive glioblastoma biomarker that downregulates MGMT expression". Neuro-Oncology. 14 (6): 712–719. doi:10.1093/neuonc/nos089. ISSN 1522-8517. PMC 3367855. PMID 22570426.
- Chen HY, Shao CJ, Chen FR, Kwan AL, Chen ZP (April 2010). "Role of ERCC1 promoter hypermethylation in drug resistance to cisplatin in human gliomas". International Journal of Cancer. 126 (8): 1944–1954. doi:10.1002/ijc.24772. PMID 19626585.
- Gao, Dan; Herman, James G.; Guo, Mingzhou (7 March 2016). "The clinical value of aberrant epigenetic changes of DNA damage repair genes in human cancer". Oncotarget. 7 (24): 37331–37346. doi:10.18632/oncotarget.7949. ISSN 1949-2553. PMC 5095080. PMID 26967246.
- Narayanan L, Fritzell JA, Baker SM, Liskay RM, Glazer PM (April 1997). "Elevated levels of mutation in multiple tissues of mice deficient in the DNA mismatch repair gene Pms2". Proceedings of the National Academy of Sciences of the United States of America. 94 (7): 3122–7. Bibcode:1997PNAS...94.3122N. doi:10.1073/pnas.94.7.3122. PMC 20332. PMID 9096356.
- Hegan DC, Narayanan L, Jirik FR, Edelmann W, Liskay RM, Glazer PM (December 2006). "Differing patterns of genetic instability in mice deficient in the mismatch repair genes Pms2, Mlh1, Msh2, Msh3 and Msh6". Carcinogenesis. 27 (12): 2402–8. doi:10.1093/carcin/bgl079. PMC 2612936. PMID 16728433.
- Tutt AN, van Oostrom CT, Ross GM, van Steeg H, Ashworth A (March 2002). "Disruption of Brca2 increases the spontaneous mutation rate in vivo: synergism with ionizing radiation". EMBO Reports. 3 (3): 255–60. doi:10.1093/embo-reports/kvf037. PMC 1084010. PMID 11850397.
- Molenaar RJ, Radivoyevitch T, Maciejewski JP, van Noorden CJ, Bleeker FE (December 2014). "The driver and passenger effects of isocitrate dehydrogenase 1 and 2 mutations in oncogenesis and survival prolongation". Biochimica et Biophysica Acta (BBA) - Reviews on Cancer. 1846 (2): 326–41. doi:10.1016/j.bbcan.2014.05.004. PMID 24880135.
- Cohen AL, Holmen SL, Colman H (May 2013). "IDH1 and IDH2 mutations in gliomas". Current Neurology and Neuroscience Reports. 13 (5): 345. doi:10.1007/s11910-013-0345-4. PMC 4109985. PMID 23532369.
- Wang P, Dong Q, Zhang C, Kuan PF, Liu Y, Jeck WR, et al. (June 2013). "Mutations in isocitrate dehydrogenase 1 and 2 occur frequently in intrahepatic cholangiocarcinomas and share hypermethylation targets with glioblastomas". Oncogene. 32 (25): 3091–100. doi:10.1038/onc.2012.315. PMC 3500578. PMID 22824796.
- Toyota M, Ahuja N, Ohe-Toyota M, Herman JG, Baylin SB, Issa JP (July 1999). "CpG island methylator phenotype in colorectal cancer". Proceedings of the National Academy of Sciences of the United States of America. 96 (15): 8681–6. Bibcode:1999PNAS...96.8681T. doi:10.1073/pnas.96.15.8681. PMC 17576. PMID 10411935.
- Nazemalhosseini Mojarad E, Kuppen PJ, Aghdaei HA, Zali MR (2013). "The CpG island methylator phenotype (CIMP) in colorectal cancer". Gastroenterology and Hepatology from Bed to Bench. 6 (3): 120–8. PMC 4017514. PMID 24834258.
- Molenaar RJ, Botman D, Smits MA, Hira VV, van Lith SA, Stap J, et al. (November 2015). "Radioprotection of IDH1-Mutated Cancer Cells by the IDH1-Mutant Inhibitor AGI-5198". Cancer Research. 75 (22): 4790–802. doi:10.1158/0008-5472.CAN-14-3603. PMID 26363012.
- Watanabe T, Nobusawa S, Kleihues P, Ohgaki H (April 2009). "IDH1 mutations are early events in the development of astrocytomas and oligodendrogliomas". The American Journal of Pathology. 174 (4): 1149–53. doi:10.2353/ajpath.2009.080958. PMC 2671348. PMID 19246647.
- Maher EA, Furnari FB, Bachoo RM, Rowitch DH, Louis DN, Cavenee WK, DePinho RA (June 2001). "Malignant glioma: genetics and biology of a grave matter". Genes & Development. 15 (11): 1311–33. doi:10.1101/gad.891601. PMID 11390353.
- Pasqualini, Claudia; Kozaki, Tatsuya; Bruschi, Marco; Nguyen, Thi Hai Hoa; Minard-Colin, Véronique; Castel, David; Grill, Jacques; Ginhoux, Florent (December 2020). "Modeling the Interaction between the Microenvironment and Tumor Cells in Brain Tumors". Neuron. 108 (6): 1025–1044. doi:10.1016/j.neuron.2020.09.018. ISSN 0896-6273. PMID 33065047. S2CID 222413763.
- von Deimling A, Eibl RH, Ohgaki H, Louis DN, von Ammon K, Petersen I, et al. (May 1992). "p53 mutations are associated with 17p allelic loss in grade II and grade III astrocytoma". Cancer Research. 52 (10): 2987–90. PMID 1349850.
- Yan H, Parsons DW, Jin G, McLendon R, Rasheed BA, Yuan W, et al. (February 2009). "IDH1 and IDH2 mutations in gliomas". The New England Journal of Medicine. 360 (8): 765–73. doi:10.1056/NEJMoa0808710. PMC 2820383. PMID 19228619.
- "Gliomas". Johns Hopkins Medicine Health Library. Retrieved 19 April 2017.
- Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, et al. (June 2016). "The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary". Acta Neuropathologica. 131 (6): 803–20. doi:10.1007/s00401-016-1545-1. PMID 27157931.
- Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, eds. (2016). WHO classification of tumours of the central nervous system. World Health Organization (Revised 4th ed.). Lyon: International Agency for Research on Cancer. ISBN 9789283244929. OCLC 951745876.
- Neuro-Oncology, Volume 25, Issue Supplement_1, June 2023, Pages i38–i39, https://doi.org/10.1093/neuonc/noad073.152
- Fyllingen, Even Hovig; Bø, Lars Eirik; Reinertsen, Ingerid; Jakola, Asgeir Store; Sagberg, Lisa Millgård; Berntsen, Erik Magnus; Salvesen, Øyvind; Solheim, Ole (1 July 2021). "Survival of glioblastoma in relation to tumor location: a statistical tumor atlas of a population-based cohort". Acta Neurochirurgica. 163 (7): 1895–1905. doi:10.1007/s00701-021-04802-6. ISSN 0942-0940. PMC 8195961. PMID 33742279.
- Persaud-Sharma, Dharam; Burns, Joseph; Trangle, Jeran; Moulik, Sabyasachi (September 2017). "Disparities in Brain Cancer in the United States: A Literature Review of Gliomas". Medical Sciences. 5 (3): 16. doi:10.3390/medsci5030016. ISSN 2076-3271. PMC 5635804. PMID 29099032.
- Liu, Huanbing; Qin, Xiaowei; Zhao, Liyan; Zhao, Gang; Wang, Yubo (2021). "Epidemiology and Survival of Patients With Brainstem Gliomas: A Population-Based Study Using the SEER Database". Frontiers in Oncology. 11. doi:10.3389/fonc.2021.692097. ISSN 2234-943X. PMC 8237753. PMID 34195093.
- Tan AC, et al. (July 2020). "Management of glioblastoma: State of the art and future directions". CA: A Cancer Journal for Clinicians. 70 (4): 299–312. doi:10.3322/caac.21613. PMID 32478924.
- Hart MG, Garside R, Rogers G, Stein K, Grant R (April 2013). "Temozolomide for high grade glioma". The Cochrane Database of Systematic Reviews. 4 (4): CD007415. doi:10.1002/14651858.CD007415.pub2. PMC 6457743. PMID 23633341.
- Platten M, Bunse L, Wick W, Bunse T (October 2016). "Concepts in glioma immunotherapy". Cancer Immunology, Immunotherapy. 65 (10): 1269–75. doi:10.1007/s00262-016-1874-x. PMID 27460064. S2CID 22635893.
- Patel MA, Pardoll DM (July 2015). "Concepts of immunotherapy for glioma". Journal of Neuro-Oncology. 123 (3): 323–30. doi:10.1007/s11060-015-1810-5. PMC 4498978. PMID 26070552.
- Suryawanshi YR, Schulze AJ (July 2021). "Oncolytic Viruses for Malignant Glioma: On the Verge of Success?". Viruses. 13 (7): 1294. doi:10.3390/v13071294. PMC 8310195. PMID 34372501.
- Wong ET, Brem S (October 2007). "Taming glioblastoma: targeting angiogenesis". Journal of Clinical Oncology. 25 (30): 4705–6. doi:10.1200/JCO.2007.13.1037. PMID 17947716. S2CID 6164155.
- Jiang B, Chaichana K, Veeravagu A, Chang SD, Black KL, Patil CG (April 2017). "Biopsy versus resection for the management of low-grade gliomas". The Cochrane Database of Systematic Reviews. 4 (6): CD009319. doi:10.1002/14651858.CD009319.pub3. PMC 6478300. PMID 28447767.
- Hart MG, Grant GR, Solyom EF, Grant R (June 2019). "Biopsy versus resection for high-grade glioma". The Cochrane Database of Systematic Reviews. 2019 (6): CD002034. doi:10.1002/14651858.CD002034.pub2. PMC 6553559. PMID 31169915.
- Glioma Meta-Analysis Trialists (GMT) Group; et al. (Glioma Meta-analysis Trialists Group) (2002). Stewart, Lesley (ed.). "Chemotherapy for high-grade glioma". The Cochrane Database of Systematic Reviews (4): CD003913. doi:10.1002/14651858.CD003913. PMID 12519620.
- Lawrie TA, Gillespie D, Dowswell T, Evans J, Erridge S, Vale L, et al. (August 2019). "Long-term neurocognitive and other side effects of radiotherapy, with or without chemotherapy, for glioma". The Cochrane Database of Systematic Reviews. 8 (8): CD013047. doi:10.1002/14651858.cd013047.pub2. PMC 6699681. PMID 31425631.
- Parasramka S, Talari G, Rosenfeld M, Guo J, Villano JL (July 2017). "Procarbazine, lomustine and vincristine for recurrent high-grade glioma". The Cochrane Database of Systematic Reviews. 2017 (7): CD011773. doi:10.1002/14651858.cd011773.pub2. PMC 6483418. PMID 28744879.
- Johnson BE, Mazor T, Hong C, Barnes M, Aihara K, McLean CY, et al. (January 2014). "Mutational analysis reveals the origin and therapy-driven evolution of recurrent glioma". Science. 343 (6167): 189–193. Bibcode:2014Sci...343..189J. doi:10.1126/science.1239947. PMC 3998672. PMID 24336570.
- "Recurrent Brain Cancers Follow Distinctive Genetic Paths". University of California Santa Cruz. University of California San Francisco. Retrieved 17 June 2015.
- Sanai N, Chang S, Berger MS (November 2011). "Low-grade gliomas in adults". Journal of Neurosurgery. 115 (5): 948–65. doi:10.3171/2011.7.JNS101238. PMID 22043865.
- Smoll NR, Gautschi OP, Schatlo B, Schaller K, Weber DC (August 2012). "Relative survival of patients with supratentorial low-grade gliomas". Neuro-Oncology. 14 (8): 1062–9. doi:10.1093/neuonc/nos144. PMC 3408266. PMID 22773277.
- Ohgaki H, Kleihues P (June 2005). "Population-based studies on incidence, survival rates, and genetic alterations in astrocytic and oligodendroglial gliomas". Journal of Neuropathology and Experimental Neurology. 64 (6): 479–89. doi:10.1093/jnen/64.6.479. PMID 15977639.
- Olson JD, Riedel E, DeAngelis LM (April 2000). "Long-term outcome of low-grade oligodendroglioma and mixed glioma". Neurology. 54 (7): 1442–8. doi:10.1212/WNL.54.7.1442. PMID 10751254. S2CID 26335770.
- Claus, Elizabeth B.; Walsh, Kyle M.; Wiencke, John K.; Molinaro, Annette M.; Wiemels, Joseph L.; Schildkraut, Joellen M.; Bondy, Melissa L.; Berger, Mitchel; Jenkins, Robert; Wrensch, Margaret (January 2015). "Survival and low-grade glioma: the emergence of genetic information". Neurosurgical Focus. 38 (1): E6. doi:10.3171/2014.10.FOCUS12367. ISSN 1092-0684. PMC 4361022. PMID 25552286.
- Bleeker FE, Molenaar RJ, Leenstra S (May 2012). "Recent advances in the molecular understanding of glioblastoma". Journal of Neuro-Oncology. 108 (1): 11–27. doi:10.1007/s11060-011-0793-0. PMC 3337398. PMID 22270850.
- Khan L, Soliman H, Sahgal A, Perry J, Xu W, Tsao MN (May 2020). "External beam radiation dose escalation for high grade glioma". Cochrane Database of Systematic Reviews. 5 (8): CD011475. doi:10.1002/14651858.CD011475.pub3. PMC 7389526. PMID 32437039.
- "Patients & Families: Basic Facts". DIPG Registry. Archived from the original on 2 May 2014. Retrieved 1 May 2014.
- Kebudi R, Cakir FB (October 2013). "Management of diffuse pontine gliomas in children: recent developments". Paediatric Drugs. 15 (5): 351–62. doi:10.1007/s40272-013-0033-5. PMID 23719782. S2CID 207491201.
- Fisher PG, Breiter SN, Carson BS, Wharam MD, Williams JA, Weingart JD, et al. (October 2000). "A clinicopathologic reappraisal of brain stem tumor classification. Identification of pilocystic astrocytoma and fibrillary astrocytoma as distinct entities". Cancer. 89 (7): 1569–76. doi:10.1002/1097-0142(20001001)89:7<1569::aid-cncr22>3.0.co;2-0. PMID 11013373. S2CID 25562391.
- Donaldson SS, Laningham F, Fisher PG (March 2006). "Advances toward an understanding of brainstem gliomas". Journal of Clinical Oncology. 24 (8): 1266–72. doi:10.1200/jco.2005.04.6599. PMID 16525181.
- Jansen MH, van Vuurden DG, Vandertop WP, Kaspers GJ (February 2012). "Diffuse intrinsic pontine gliomas: a systematic update on clinical trials and biology". Cancer Treatment Reviews. 38 (1): 27–35. doi:10.1016/j.ctrv.2011.06.007. PMID 21764221.
- Molenaar RJ, Verbaan D, Lamba S, Zanon C, Jeuken JW, Boots-Sprenger SH, et al. (September 2014). "The combination of IDH1 mutations and MGMT methylation status predicts survival in glioblastoma better than either IDH1 or MGMT alone". Neuro-Oncology. 16 (9): 1263–73. doi:10.1093/neuonc/nou005. PMC 4136888. PMID 24510240.