ERCC1
DNA excision repair protein ERCC-1 is a protein that in humans is encoded by the ERCC1 gene.[5] Together with ERCC4, ERCC1 forms the ERCC1-XPF enzyme complex that participates in DNA repair and DNA recombination.[6][7]
Many aspects of these two gene products are described together here because they are partners during DNA repair. The ERCC1-XPF nuclease is an essential activity in the pathway of DNA nucleotide excision repair (NER). The ERCC1-XPF nuclease also functions in pathways to repair double-strand breaks in DNA, and in the repair of “crosslink” damage that harmfully links the two DNA strands.
Cells with disabling mutations in ERCC1 are more sensitive than normal to particular DNA damaging agents, including ultraviolet (UV) radiation and to chemicals that cause crosslinking between DNA strands. Genetically engineered mice with disabling mutations in ERCC1 have defects in DNA repair, accompanied by metabolic stress-induced changes in physiology that result in premature aging.[8] Complete deletion of ERCC1 is incompatible with viability of mice, and no human individuals have been found with complete (homozygous) deletion of ERCC1. Rare individuals in the human population harbor inherited mutations that impair the function of ERCC1. When the normal genes are absent, these mutations can lead to human syndromes, including Cockayne syndrome (CS) and COFS.
ERCC1 and ERCC4 are the gene names assigned in mammalian genomes, including the human genome (Homo sapiens). Similar genes with similar functions are found in all eukaryotic organisms.
Gene
The genomic DNA for ERCC1 was the first human DNA repair gene to be isolated by molecular cloning. The original method was by transfer of fragments of the human genome to ultraviolet light (UV)-sensitive mutant cell lines derived from Chinese hamster ovary cells.[9] Reflecting this cross-species genetic complementation method, the gene was called “Excision repair cross-complementing 1”. Multiple independent complementation groups of Chinese hamster ovary (CHO) cells were isolated,[10] and this gene restored UV resistance to cells of complementation group 1.
The human ERCC1 gene encodes the ERCC1 protein of 297 amino acids with a molecular mass of about 32,500 daltons.
Genes similar to ERCC1 with equivalent functions (orthologs) are found in other eukaryotic genomes. Some of the most studied gene orthologs include RAD10 in the budding yeast Saccharomyces cerevisiae, and swi10+ in the fission yeast Schizosaccharomyces pombe.
Protein
One ERCC1 molecule and one XPF molecule bind together, forming an ERCC1-XPF heterodimer which is the active nuclease form of the enzyme. In the ERCC1–XPF heterodimer, ERCC1 mediates DNA– and protein–protein interactions. XPF provides the endonuclease active site and is involved in DNA binding and additional protein–protein interactions.[9]
The ERCC4/XPF protein consists of two conserved domains separated by a less conserved region in the middle. The N-terminal region has homology to several conserved domains of DNA helicases belonging to superfamily II, although XPF is not a DNA helicase.[11] The C-terminal region of XPF includes the active site residues for nuclease activity.[12] Most of the ERCC1 protein is related at the sequence level to the C-terminus of the XPF protein,[13] but residues in the nuclease domain are not present. A DNA binding “helix-hairpin-helix” domain at the C-terminus of each protein.
By primary sequence and protein structural similarity, the ERCC1-XPF nuclease is a member of a broader family of structure specific DNA nucleases comprising two subunits. Such nucleases include, for example, the MUS81-EME1 nuclease.
Structure-specific nuclease
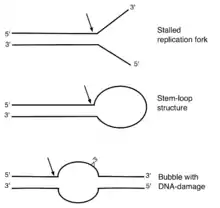
The ERCC1–XPF complex is a structure-specific endonuclease. ERCC1-XPF does not cut DNA that is exclusively single-stranded or double-stranded, but it cleaves the DNA phosphodiester backbone specifically at junctions between double-stranded and single-stranded DNA. It introduces a cut in double-stranded DNA on the 5′ side of such a junction, about two nucleotides away.[14] This structure-specificity was initially demonstrated for RAD10-RAD1, the yeast orthologs of ERCC1 and XPF.[15]
The hydrophobic helix–hairpin–helix motifs in the C-terminal regions of ERCC1 and XPF interact to promote dimerization of the two proteins.[16] There is no catalytic activity in the absence of dimerization. Indeed, although the catalytic domain is within XPF and ERCC1 is catalytically inactive, ERCC1 is indispensable for activity of the complex.
Several models have been proposed for binding of ERCC1–XPF to DNA, based on partial structures of relevant protein fragments at atomic resolution.[16] DNA binding mediated by the helix-hairpin-helix domains of ERCC1 and XPF domains positions the heterodimer at the junction between double-stranded and single-stranded DNA.
Nucleotide excision repair
During nucleotide excision repair, several protein complexes cooperate to recognize damaged DNA and locally separate the DNA helix for a short distance on either side of the site of a DNA damage. The ERCC1–XPF nuclease incises the damaged DNA strand on the 5′ side of the lesion.[14] During NER, the ERCC1 protein interacts with the XPA protein to coordinate DNA and protein binding.
DNA double-strand break repair
Mammalian cells with mutant ERCC1–XPF are moderately more sensitive than normal cells to agents (such as ionizing radiation) that cause double-stranded breaks in DNA.[17][18] Particular pathways of both homologous recombination repair and non-homologous end-joining rely on ERCC1-XPF function.[19][20] The relevant activity of ERCC1–XPF for both types of double-strand break repair is the ability to remove non-homologous 3′ single-stranded tails from DNA ends before rejoining. This activity is needed during a single-strand annealing subpathway of homologous recombination. Trimming of 3’ single-stranded tail is also needed in a mechanistically distinct subpathway of non-homologous end-joining, dependent on the Ku proteins.[17] Homologous integration of DNA, an important technique for genetic manipulation, is dependent on the function of ERCC1-XPF in the host cell.[21]
DNA interstrand crosslink repair
Mammalian cells carrying mutations in ERCC1 or XPF are especially sensitive to agents that cause DNA interstrand crosslinks.[22] Interstrand crosslinks block the progression of DNA replication, and structures at blocked DNA replication forks provide substrates for cleavage by ERCC1-XPF.[23][24] Incisions may be made on either side of the crosslink on one DNA strand to unhook the crosslink and initiate repair. Alternatively, a double-strand break may be made in the DNA near the ICL, and subsequent homologous recombination repair may involve ERCC1-XPF action. Although not the only nuclease involved, ERCC1–XPF is required for ICL repair during several phases of the cell cycle.[25][26]
Clinical significance
Cerebro-oculo-facio-skeletal syndrome
A few patients with severely disabling ERCC1 mutations that cause cerebro-oculo-facio-skeletal syndrome (COFS) have been reported.[8][27] COFS syndrome is a rare recessive disorder in which affected individuals undergo rapid neurologic decline and indications of accelerated aging. A very severe case of such disabling mutations is F231L mutation in the tandem helix-hairpin-helix domain of ERCC1 at its interface with XPF.[27][28] It is shown that this single mutation is very important for the stability of the ERCC1-XPF complex. This Phenylalanine residue is assisting ERCC1 to accommodate a key Phenylalanine residue from XPF (F894) and the mutation (F231L) disturbs this accommodating function. As a consequence, F894 protrudes out of the interface and the mutant complex is dissociating faster compared to the native one.[28] The life span of patients with such mutations is often around 1–2 years.[27]
Cockayne syndrome
One Cockayne syndrome (CS) type II patient designated CS20LO exhibited a homozygous mutation in exon 7 of ERCC1, producing a F231L mutation.[29]
Relevance in chemotherapy
Measuring ERCC1 activity may have utility in clinical cancer medicine because one mechanism of resistance to platinum chemotherapy drugs correlates with high ERCC1 activity. Nucleotide excision repair (NER) is the primary DNA repair mechanism that removes the therapeutic platinum-DNA adducts from the tumor DNA. ERCC1 activity levels, being an important part of the NER common final pathway, may serve as a marker of general NER throughput. This has been suggested for patients with gastric,[30] ovarian and bladder cancers.[31] In Non-small cell lung carcinoma (NSCLC), surgically removed tumors that receive no further therapy have a better survival if ERCC1-positive than if ERCC1-negative. Thus, ERCC1 positivity is a favorable prognostic marker, referring to how the disease will proceed if not further treated. ERCC1-positive NSCLC tumors do not benefit from adjuvant platinum chemotherapy. However, ERCC1-negative NSCLC tumors, prognostically worse without treatment, derive substantial benefit from adjuvant cisplatin-based chemotherapy. High ERCC1 is thus a negative predictive marker, referring to how it will respond to a specific type of treatment.[32][33] In colorectal cancer, clinical trials have not demonstrated the predictive ability of ERCC1 in oxaliplatin‐based treatment. Thus, European Society for Medical Oncology (ESMO) has not recommended ERCC1 testing prior to the use of oxaliplatin in routine practice.[34][35] ERCC1 genotyping in humans has shown significant polymorphism at codon 118.[36] These polymorphisms may have differential effects on platinum and mitomycin damage.[36]
Deficiency in cancer
ERCC1 protein expression is reduced or absent in 84% to 100% of colorectal cancers,[37][38] and lower expression of ERCC1 has been reported as being associated with unfavorable prognosis in patients undergoing treatment with oxaliplatin.[34] The promoter of ERCC1 is methylated in 38% of gliomas, resulting in reduced mRNA and protein expression.[39] The promoter of ERCC1 was located in the DNA 5 kilobases upstream of the protein coding region.[39] Frequencies of epigenetic reductions of nine other DNA repair genes have been evaluated in various cancers and range from 2% (OGG1 in papillary thyroid cancer) to 88% and 90% (MGMT in gastric and colon cancers, respectively). Thus, reduction of protein expression of ERCC1 in 84% to 100% of colon cancers indicates that reduced ERCC1 is one of the most frequent reductions of a DNA repair gene observed in a cancer. Deficiency in ERCC1 protein expression appears to be an early event in colon carcinogenesis, since ERCC1 was found to be deficient in 40% of the crypts within 10 cm on each side of colonic adenocarcinomas (within the early field defects from which the cancers likely arose).[37]
Cadmium (Cd) and its compounds are well-known human carcinogens. During Cd-induced malignant transformation, the promoter regions of ERCC1, as well as of hMSH2, XRCC1, and hOGG1, were heavily methylated and both the messenger RNA and proteins of these DNA repair genes were progressively reduced.[40] DNA damage also increased with Cd-induced transformation.[40] Reduction of protein expression of ERCC1 in progression to sporadic cancer is unlikely to be due to mutation. While germ line (familial) mutations in DNA repair genes cause a high risk of cancer (see inherited impairment in DNA repair increases cancer risk), somatic mutations in DNA repair genes, including ERCC1, only occur at low levels in sporadic (non-familial) cancers.[41]
Control of ERCC1 protein level occurred at the translational level. In addition to the wild-type sequence, three splice variants of mRNA ERCC1 exist.[42] ERCC1 mRNA is also found to have either wild-type or three alternative transcription start points. Neither the level of overall mRNA transcription, splice variation nor transcription start point of mRNA correlates with protein level of ERCC1. The rate of ERCC1 protein turnover also does not correlate with ERCC1 protein level. A translational level control of ERCC1, due to a microRNA (miRNA), has been shown during HIV viral infection. A trans-activation response element (TAR) miRNA, coded for by the HIV virus, down-regulates ERCC1 protein expression.[43] TAR miRNA allows ERCC1 mRNA to be transcribed, but acts at the p-body level to prevent translation of ERCC1 protein. (A p-body is a cytoplasmic granule “processing body” that interacts with miRNAs to repress translation or trigger degradation of target RNAs.) In breast cancer cell lines, almost one third (55/167) of miRNA promoters were targets for aberrant methylation (epigenetic repression).[44] In breast cancers themselves, methylation of let-7a-3/let-7b miRNA in particular was found. This indicates that let-7a-3/let-7b can be epigenetically repressed.
Repression of let-7a can cause repression of ERCC1 expression through an intermediary step involving the HMGA2 gene. The let-7a miRNA normally represses the HMGA2 gene, and in normal adult tissues, almost no HMGA2 protein is present.[45] (See also Let-7 microRNA precursor.) Reduction or absence of let-7a miRNA allows high expression of the HMGA2 protein. HMGA proteins are characterized by three DNA-binding domains, called AT-hooks, and an acidic carboxy-terminal tail. HMGA proteins are chromatin architectural transcription factors that both positively and negatively regulate the transcription of a variety of genes. They do not display direct transcriptional activation capacity, but regulate gene expression by changing local DNA conformation. Regulation is achieved by binding to AT-rich regions in the DNA and/or direct interaction with several transcription factors.[46] HMGA2 targets and modifies the chromatin architecture at the ERCC1 gene, reducing its expression.[47] Hypermethylation of the promoter for let-7a miRNA reduces its expression and this allows hyperexpression of HMGA2. Hyperexpression of HMGA2 can then reduce expression of ERCC1.
Thus, there are three mechanisms that may be responsible for the low level of protein expression of ERCC1 in 84% to 100% of sporadic colon cancers. From results in gliomas and in cadmium carcinogenesis, methylation of the ERCC1 promoter may be a factor. One or more miRNAs that repress ERCC1 may be a factor. And epigenetically reduced let-7a miRNA allowing hyperexpression of HMGA2 could also reduce protein expression of ERCC1 in colon cancers. Which epigenetic mechanism occurs most frequently, or whether multiple epigenetic mechanisms reduce ERCC1 protein expression in colon cancers has not been determined.
Accelerated aging
DNA repair-deficient Ercc1 mutant mice show numerous features of accelerated aging, and have a limited lifespan.[48] Accelerated aging in the mutant involves various organs. Ercc1 mutant mice are deficient in several DNA repair processes including transcription-coupled DNA repair. This deficiency prevents resumption of RNA synthesis on the template DNA strand subsequent to it receiving a transcription-blocking DNA damage. Such blockages of transcription appear to promote premature aging, particularly in non-proliferating or slowly proliferating organs such as the nervous system, liver and kidney[49] (see DNA damage theory of aging).
When Ercc1 mutant mice were subjected to dietary restriction their response closely resembled the beneficial response to dietary restriction of wild-type mice. Dietary restriction extended the lifespan of the Ercc1 mutant mice from 10 to 35 weeks for males and from 13 to 39 weeks for females.[48] It appears that in Ercc1 mutant mice dietary restriction while delaying aging also attenuates accumulation of genome-wide DNA damage and preserves transcriptional output, likely contributing to improved cell viability.[48]
Spermatogenesis and oogenesis
Both male and female Ercc1-deficient mice are infertile.[50] The DNA repair function of Ercc1 appears to be required in both male and female germ cells at all stages of their maturation. The testes of Ercc1-deficient mice have an increased level of 8-oxoguanine in their DNA, suggesting that Ercc1 may have a role in removing oxidative DNA damages.
Notes
References
- GRCh38: Ensembl release 89: ENSG00000012061 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000003549 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- Westerveld A, Hoeijmakers JH, van Duin M, de Wit J, Odijk H, Pastink A, et al. (Sep 1984). "Molecular cloning of a human DNA repair gene". Nature. 310 (5976): 425–9. Bibcode:1984Natur.310..425W. doi:10.1038/310425a0. PMID 6462228. S2CID 4336902.
- Friedberg EC, Walker GC, Siede W, Wood RD, Schultz RA, Ellenberger T, eds. (2006). DNA Repair and Mutagenesis. ASM Press. p. 286. ISBN 978-1555813192.
- "Entrez Gene: ERCC4 excision repair cross-complementing rodent repair deficiency, complementation group 4".
- Gregg SQ, Robinson AR, Niedernhofer LJ (July 2011). "Physiological consequences of defects in ERCC1-XPF DNA repair endonuclease". DNA Repair. 10 (7): 781–91. doi:10.1016/j.dnarep.2011.04.026. PMC 3139823. PMID 21612988.
- Westerveld A, Hoeijmakers JH, van Duin M, de Wit J, Odijk H, Pastink A, et al. (1984). "Molecular cloning of a human DNA repair gene". Nature. 310 (5976): 425–9. Bibcode:1984Natur.310..425W. doi:10.1038/310425a0. PMID 6462228. S2CID 4336902.
- Busch D, Greiner C, Lewis K, Ford R, Adair G, Thompson L (September 1989). "Summary of complementation groups of UV-sensitive CHO cell mutants isolated by large-scale screening". Mutagenesis. 4 (5): 349–54. doi:10.1093/mutage/4.5.349. PMID 2687628.
- Sgouros J, Gaillard PH, Wood RD (March 1999). "A relationship between a DNA-repair/recombination nuclease family and archaeal helicases". Trends in Biochemical Sciences. 24 (3): 95–7. doi:10.1016/s0968-0004(99)01355-9. PMID 10203755.
- Enzlin JH, Schärer OD (April 2002). "The active site of the DNA repair endonuclease XPF-ERCC1 forms a highly conserved nuclease motif". The EMBO Journal. 21 (8): 2045–53. doi:10.1093/emboj/21.8.2045. PMC 125967. PMID 11953324.
- Gaillard PH, Wood RD (February 2001). "Activity of individual ERCC1 and XPF subunits in DNA nucleotide excision repair". Nucleic Acids Research. 29 (4): 872–9. doi:10.1093/nar/29.4.872. PMC 29621. PMID 11160918.
- Sijbers AM, de Laat WL, Ariza RR, Biggerstaff M, Wei YF, Moggs JG, et al. (September 1996). "Xeroderma pigmentosum group F caused by a defect in a structure-specific DNA repair endonuclease" (PDF). Cell. 86 (5): 811–22. doi:10.1016/s0092-8674(00)80155-5. hdl:1765/3110. PMID 8797827. S2CID 12957716.
- Bardwell AJ, Bardwell L, Tomkinson AE, Friedberg EC (September 1994). "Specific cleavage of model recombination and repair intermediates by the yeast Rad1-Rad10 DNA endonuclease". Science. 265 (5181): 2082–5. Bibcode:1994Sci...265.2082B. doi:10.1126/science.8091230. PMID 8091230.
- McNeil EM, Melton DW (November 2012). "DNA repair endonuclease ERCC1-XPF as a novel therapeutic target to overcome chemoresistance in cancer therapy". Nucleic Acids Research. 40 (20): 9990–10004. doi:10.1093/nar/gks818. PMC 3488251. PMID 22941649.
- Ahmad A, Robinson AR, Duensing A, van Drunen E, Beverloo HB, Weisberg DB, et al. (August 2008). "ERCC1-XPF endonuclease facilitates DNA double-strand break repair". Molecular and Cellular Biology. 28 (16): 5082–92. doi:10.1128/MCB.00293-08. PMC 2519706. PMID 18541667.
- Wood RD, Burki HJ, Hughes M, Poley A (February 1983). "Radiation-induced lethality and mutation in a repair-deficient CHO cell line". International Journal of Radiation Biology and Related Studies in Physics, Chemistry and Medicine. 43 (2): 207–13. doi:10.1080/09553008314550241. PMID 6600735.
- Al-Minawi AZ, Saleh-Gohari N, Helleday T (January 2008). "The ERCC1/XPF endonuclease is required for efficient single-strand annealing and gene conversion in mammalian cells". Nucleic Acids Research. 36 (1): 1–9. doi:10.1093/nar/gkm888. PMC 2248766. PMID 17962301.
- Sargent RG, Rolig RL, Kilburn AE, Adair GM, Wilson JH, Nairn RS (November 1997). "Recombination-dependent deletion formation in mammalian cells deficient in the nucleotide excision repair gene ERCC1". Proceedings of the National Academy of Sciences of the United States of America. 94 (24): 13122–7. Bibcode:1997PNAS...9413122S. doi:10.1073/pnas.94.24.13122. PMC 24273. PMID 9371810.
- Niedernhofer LJ, Essers J, Weeda G, Beverloo B, de Wit J, Muijtjens M, et al. (November 2001). "The structure-specific endonuclease Ercc1-Xpf is required for targeted gene replacement in embryonic stem cells". The EMBO Journal. 20 (22): 6540–9. doi:10.1093/emboj/20.22.6540. PMC 125716. PMID 11707424.
- Wood RD (July 2010). "Mammalian nucleotide excision repair proteins and interstrand crosslink repair". Environmental and Molecular Mutagenesis. 51 (6): 520–6. doi:10.1002/em.20569. PMC 3017513. PMID 20658645.
- Klein Douwel D, Boonen RA, Long DT, Szypowska AA, Räschle M, Walter JC, Knipscheer P (May 2014). "XPF-ERCC1 acts in Unhooking DNA interstrand crosslinks in cooperation with FANCD2 and FANCP/SLX4". Molecular Cell. 54 (3): 460–71. doi:10.1016/j.molcel.2014.03.015. PMC 5067070. PMID 24726325.
- Kuraoka I, Kobertz WR, Ariza RR, Biggerstaff M, Essigmann JM, Wood RD (August 2000). "Repair of an interstrand DNA cross-link initiated by ERCC1-XPF repair/recombination nuclease". The Journal of Biological Chemistry. 275 (34): 26632–6. doi:10.1074/jbc.C000337200. PMID 10882712.
- Clauson C, Schärer OD, Niedernhofer L (October 2013). "Advances in understanding the complex mechanisms of DNA interstrand cross-link repair". Cold Spring Harbor Perspectives in Biology. 5 (10): a012732. doi:10.1101/cshperspect.a012732. PMC 4123742. PMID 24086043.
- Rahn JJ, Adair GM, Nairn RS (July 2010). "Multiple roles of ERCC1-XPF in mammalian interstrand crosslink repair". Environmental and Molecular Mutagenesis. 51 (6): 567–81. doi:10.1002/em.20583. PMID 20658648. S2CID 29240680.
- Jaspers NG, Raams A, Silengo MC, Wijgers N, Niedernhofer LJ, Robinson AR, et al. (March 2007). "First reported patient with human ERCC1 deficiency has cerebro-oculo-facio-skeletal syndrome with a mild defect in nucleotide excision repair and severe developmental failure". American Journal of Human Genetics. 80 (3): 457–66. doi:10.1086/512486. PMC 1821117. PMID 17273966.
- Faridounnia M, Wienk H, Kovačič L, Folkers GE, Jaspers NG, Kaptein R, et al. (August 2015). "The Cerebro-oculo-facio-skeletal Syndrome Point Mutation F231L in the ERCC1 DNA Repair Protein Causes Dissociation of the ERCC1-XPF Complex". The Journal of Biological Chemistry. 290 (33): 20541–55. doi:10.1074/jbc.M114.635169. PMC 4536458. PMID 26085086.
- Kashiyama K, Nakazawa Y, Pilz DT, Guo C, Shimada M, Sasaki K, et al. (May 2013). "Malfunction of nuclease ERCC1-XPF results in diverse clinical manifestations and causes Cockayne syndrome, xeroderma pigmentosum, and Fanconi anemia". American Journal of Human Genetics. 92 (5): 807–19. doi:10.1016/j.ajhg.2013.04.007. PMC 3644632. PMID 23623389.
- Kwon HC, Roh MS, Oh SY, Kim SH, Kim MC, Kim JS, Kim HJ (March 2007). "Prognostic value of expression of ERCC1, thymidylate synthase, and glutathione S-transferase P1 for 5-fluorouracil/oxaliplatin chemotherapy in advanced gastric cancer". Annals of Oncology. 18 (3): 504–9. doi:10.1093/annonc/mdl430. PMID 17322540.
- Bellmunt J, Paz-Ares L, Cuello M, Cecere FL, Albiol S, Guillem V, et al. (March 2007). "Gene expression of ERCC1 as a novel prognostic marker in advanced bladder cancer patients receiving cisplatin-based chemotherapy". Annals of Oncology. 18 (3): 522–8. doi:10.1093/annonc/mdl435. PMID 17229776.
- Olaussen KA, Dunant A, Fouret P, Brambilla E, André F, Haddad V, et al. (September 2006). "DNA repair by ERCC1 in non-small-cell lung cancer and cisplatin-based adjuvant chemotherapy". The New England Journal of Medicine. 355 (10): 983–91. doi:10.1056/NEJMoa060570. PMID 16957145.
- Soria JC (July 2007). "ERCC1-tailored chemotherapy in lung cancer: the first prospective randomized trial". Journal of Clinical Oncology. 25 (19): 2648–9. doi:10.1200/JCO.2007.11.3167. PMID 17602070.
- Yau TO (October 2019). "Precision treatment in colorectal cancer: Now and the future". JGH Open. 3 (5): 361–369. doi:10.1002/jgh3.12153. PMC 6788378. PMID 31633039.
- Van Cutsem E, Cervantes A, Adam R, Sobrero A, Van Krieken JH, Aderka D, et al. (August 2016). "ESMO consensus guidelines for the management of patients with metastatic colorectal cancer". Annals of Oncology. 27 (8): 1386–422. doi:10.1093/annonc/mdw235. PMID 27380959.
- Bohanes P, Labonte MJ, Lenz HJ (September 2011). "A review of excision repair cross-complementation group 1 in colorectal cancer". Clinical Colorectal Cancer. 10 (3): 157–64. doi:10.1016/j.clcc.2011.03.024. PMID 21855036.
- Facista A, Nguyen H, Lewis C, Prasad AR, Ramsey L, Zaitlin B, et al. (April 2012). "Deficient expression of DNA repair enzymes in early progression to sporadic colon cancer". Genome Integrity. 3 (1): 3. doi:10.1186/2041-9414-3-3. PMC 3351028. PMID 22494821.
- Smith DH, Fiehn AM, Fogh L, Christensen IJ, Hansen TP, Stenvang J, et al. (March 2014). "Measuring ERCC1 protein expression in cancer specimens: validation of a novel antibody". Scientific Reports. 4: 4313. Bibcode:2014NatSR...4E4313S. doi:10.1038/srep04313. PMC 3945488. PMID 24603753.
- Chen HY, Shao CJ, Chen FR, Kwan AL, Chen ZP (April 2010). "Role of ERCC1 promoter hypermethylation in drug resistance to cisplatin in human gliomas". International Journal of Cancer. 126 (8): 1944–1954. doi:10.1002/ijc.24772. PMID 19626585. S2CID 3423262.
- Zhou ZH, Lei YX, Wang CX (February 2012). "Analysis of aberrant methylation in DNA repair genes during malignant transformation of human bronchial epithelial cells induced by cadmium". Toxicological Sciences. 125 (2): 412–7. doi:10.1093/toxsci/kfr320. PMID 22112500.
- Wood LD, Parsons DW, Jones S, Lin J, Sjöblom T, Leary RJ, et al. (November 2007). "The genomic landscapes of human breast and colorectal cancers". Science. 318 (5853): 1108–13. Bibcode:2007Sci...318.1108W. CiteSeerX 10.1.1.218.5477. doi:10.1126/science.1145720. PMID 17932254. S2CID 7586573.
- McGurk CJ, Cummings M, Köberle B, Hartley JA, Oliver RT, Masters JR (April 2006). "Regulation of DNA repair gene expression in human cancer cell lines". Journal of Cellular Biochemistry. 97 (5): 1121–36. doi:10.1002/jcb.20711. PMID 16315315. S2CID 24969413.
- Klase Z, Winograd R, Davis J, Carpio L, Hildreth R, Heydarian M, et al. (February 2009). "HIV-1 TAR miRNA protects against apoptosis by altering cellular gene expression". Retrovirology. 6: 18. doi:10.1186/1742-4690-6-18. PMC 2654423. PMID 19220914.
- Vrba L, Muñoz-Rodríguez JL, Stampfer MR, Futscher BW (2013). "miRNA gene promoters are frequent targets of aberrant DNA methylation in human breast cancer". PLOS ONE. 8 (1): e54398. Bibcode:2013PLoSO...854398V. doi:10.1371/journal.pone.0054398. PMC 3547033. PMID 23342147.
- Motoyama K, Inoue H, Nakamura Y, Uetake H, Sugihara K, Mori M (April 2008). "Clinical significance of high mobility group A2 in human gastric cancer and its relationship to let-7 microRNA family". Clinical Cancer Research. 14 (8): 2334–40. doi:10.1158/1078-0432.CCR-07-4667. PMID 18413822.
- Cleynen I, Van de Ven WJ (February 2008). "The HMGA proteins: a myriad of functions (Review)". International Journal of Oncology. 32 (2): 289–305. doi:10.3892/ijo.32.2.289. PMID 18202751.
- Borrmann L, Schwanbeck R, Heyduk T, Seebeck B, Rogalla P, Bullerdiek J, Wisniewski JR (December 2003). "High mobility group A2 protein and its derivatives bind a specific region of the promoter of DNA repair gene ERCC1 and modulate its activity". Nucleic Acids Research. 31 (23): 6841–51. doi:10.1093/nar/gkg884. PMC 290254. PMID 14627817.
- Vermeij WP, Dollé ME, Reiling E, Jaarsma D, Payan-Gomez C, Bombardieri CR, et al. (September 2016). "Restricted diet delays accelerated ageing and genomic stress in DNA-repair-deficient mice". Nature. 537 (7620): 427–431. Bibcode:2016Natur.537..427V. doi:10.1038/nature19329. PMC 5161687. PMID 27556946.
- Marteijn JA, Lans H, Vermeulen W, Hoeijmakers JH (July 2014). "Understanding nucleotide excision repair and its roles in cancer and ageing". Nature Reviews. Molecular Cell Biology. 15 (7): 465–81. doi:10.1038/nrm3822. PMID 24954209. S2CID 9174323.
- Hsia KT, Millar MR, King S, Selfridge J, Redhead NJ, Melton DW, Saunders PT (January 2003). "DNA repair gene Ercc1 is essential for normal spermatogenesis and oogenesis and for functional integrity of germ cell DNA in the mouse". Development. 130 (2): 369–78. doi:10.1242/dev.00221. PMID 12466203.
Further reading
- Olaussen KA, Mountzios G, Soria JC (July 2007). "ERCC1 as a risk stratifier in platinum-based chemotherapy for nonsmall-cell lung cancer". Current Opinion in Pulmonary Medicine. 13 (4): 284–9. doi:10.1097/MCP.0b013e32816b5c63. PMID 17534174. S2CID 23038328.
- van Duin M, de Wit J, Odijk H, Westerveld A, Yasui A, Koken MH, et al. (March 1986). "Molecular characterization of the human excision repair gene ERCC-1: cDNA cloning and amino acid homology with the yeast DNA repair gene RAD10". Cell. 44 (6): 913–23. doi:10.1016/0092-8674(86)90014-0. hdl:1765/2990. PMID 2420469. S2CID 40370483.
- van Duin M, van Den Tol J, Hoeijmakers JH, Bootsma D, Rupp IP, Reynolds P, et al. (April 1989). "Conserved pattern of antisense overlapping transcription in the homologous human ERCC-1 and yeast RAD10 DNA repair gene regions". Molecular and Cellular Biology. 9 (4): 1794–8. doi:10.1128/MCB.9.4.1794. PMC 362600. PMID 2471070.
- Hoeijmakers JH (1987). "Characterization of genes and proteins involved in excision repair of human cells". Journal of Cell Science. Supplement. 6: 111–25. doi:10.1242/jcs.1984.Supplement_6.7. PMID 2821019.
- Hoeijmakers JH, van Duin M, Westerveld A, Yasui A, Bootsma D (1987). "Identification of DNA repair genes in the human genome". Cold Spring Harbor Symposia on Quantitative Biology. 51 Pt 1 (1): 91–101. doi:10.1101/sqb.1986.051.01.012. hdl:1765/2992. PMID 3034490.
- van Duin M, van den Tol J, Warmerdam P, Odijk H, Meijer D, Westerveld A, et al. (June 1988). "Evolution and mutagenesis of the mammalian excision repair gene ERCC-1". Nucleic Acids Research. 16 (12): 5305–22. doi:10.1093/nar/16.12.5305. PMC 336769. PMID 3290851.
- Nagai A, Saijo M, Kuraoka I, Matsuda T, Kodo N, Nakatsu Y, et al. (June 1995). "Enhancement of damage-specific DNA binding of XPA by interaction with the ERCC1 DNA repair protein". Biochemical and Biophysical Research Communications. 211 (3): 960–6. doi:10.1006/bbrc.1995.1905. hdl:1765/60251. PMID 7598728.
- Li L, Elledge SJ, Peterson CA, Bales ES, Legerski RJ (May 1994). "Specific association between the human DNA repair proteins XPA and ERCC1". Proceedings of the National Academy of Sciences of the United States of America. 91 (11): 5012–6. Bibcode:1994PNAS...91.5012L. doi:10.1073/pnas.91.11.5012. PMC 43920. PMID 8197174.
- Park CH, Sancar A (May 1994). "Formation of a ternary complex by human XPA, ERCC1, and ERCC4(XPF) excision repair proteins". Proceedings of the National Academy of Sciences of the United States of America. 91 (11): 5017–21. Bibcode:1994PNAS...91.5017P. doi:10.1073/pnas.91.11.5017. PMC 43921. PMID 8197175.
- McWhir J, Selfridge J, Harrison DJ, Squires S, Melton DW (November 1993). "Mice with DNA repair gene (ERCC-1) deficiency have elevated levels of p53, liver nuclear abnormalities and die before weaning". Nature Genetics. 5 (3): 217–24. doi:10.1038/ng1193-217. PMID 8275084. S2CID 20715351.
- Trask B, Fertitta A, Christensen M, Youngblom J, Bergmann A, Copeland A, et al. (January 1993). "Fluorescence in situ hybridization mapping of human chromosome 19: cytogenetic band location of 540 cosmids and 70 genes or DNA markers". Genomics. 15 (1): 133–45. doi:10.1006/geno.1993.1021. PMID 8432525.
- Yu JJ, Mu C, Lee KB, Okamoto A, Reed EL, Bostick-Bruton F, et al. (September 1997). "A nucleotide polymorphism in ERCC1 in human ovarian cancer cell lines and tumor tissues". Mutation Research. 382 (1–2): 13–20. doi:10.1016/s1383-5726(97)00004-6. PMID 9360634.
- Hayashi T, Takao M, Tanaka K, Yasui A (June 1998). "ERCC1 mutations in UV-sensitive Chinese hamster ovary (CHO) cell lines". Mutation Research. 407 (3): 269–76. doi:10.1016/s0921-8777(98)00013-5. PMID 9653453.
- de Laat WL, Sijbers AM, Odijk H, Jaspers NG, Hoeijmakers JH (September 1998). "Mapping of interaction domains between human repair proteins ERCC1 and XPF". Nucleic Acids Research. 26 (18): 4146–52. doi:10.1093/nar/26.18.4146. PMC 147836. PMID 9722633.
- Lin YW, Kubota M, Koishi S, Sawada M, Usami I, Watanabe K, Akiyama Y (November 1998). "Analysis of mutations at the DNA repair genes in acute childhood leukaemia". British Journal of Haematology. 103 (2): 462–6. doi:10.1046/j.1365-2141.1998.00973.x. PMID 9827920. S2CID 25175169.
- Houtsmuller AB, Rademakers S, Nigg AL, Hoogstraten D, Hoeijmakers JH, Vermeulen W (May 1999). "Action of DNA repair endonuclease ERCC1/XPF in living cells". Science. 284 (5416): 958–61. Bibcode:1999Sci...284..958H. doi:10.1126/science.284.5416.958. PMID 10320375.
- Cheng L, Guan Y, Li L, Legerski RJ, Einspahr J, Bangert J, et al. (September 1999). "Expression in normal human tissues of five nucleotide excision repair genes measured simultaneously by multiplex reverse transcription-polymerase chain reaction". Cancer Epidemiology, Biomarkers & Prevention. 8 (9): 801–7. PMID 10498399.
- Yu JJ, Thornton K, Guo Y, Kotz H, Reed E (November 2001). "An ERCC1 splicing variant involving the 5'-UTR of the mRNA may have a transcriptional modulatory function". Oncogene. 20 (52): 7694–8. doi:10.1038/sj.onc.1204977. PMID 11753647.
- Li QQ, Yunmbam MK, Zhong X, Yu JJ, Mimnaugh EG, Neckers L, Reed E (2002). "Lactacystin enhances cisplatin sensitivity in resistant human ovarian cancer cell lines via inhibition of DNA repair and ERCC-1 expression". Cellular and Molecular Biology. 47 Online Pub: OL61-72. PMID 11936875.