Hafnium(IV) iodide
Hafnium(IV) iodide is the inorganic compound with the formula HfI4. It is a red-orange, moisture sensitive, sublimable solid that is produced by heating a mixture of hafnium with excess iodine.[3] It is an intermediate in the crystal bar process for producing hafnium metal.
 | |
| Names | |
|---|---|
| IUPAC name
Hafnium(IV) iodide | |
| Other names
hafnium tetraiodide, tetraiodohafnium | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.150.349 |
| EC Number |
|
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| HfI4 | |
| Molar mass | 686.11[1] |
| Appearance | red-orange[1] |
| Density | 5.60 g/cm3[1] |
| Melting point | 449 °C (840 °F; 722 K)[1] |
| Boiling point | 394 °C (741 °F; 667 K)[1] (sublimes) |
| Structure | |
| Monoclinic, mS40 | |
| C2/c, No. 15[2] | |
a = 1.1787 nm, b = 1.1801 nm, c = 1.2905 nm | |
| Related compounds | |
Other anions |
Hafnium(IV) fluoride Hafnium(IV) chloride Hafnium(IV) bromide |
Other cations |
Titanium(IV) iodide Zirconium(IV) iodide |
Related compounds |
Hafnium(III) iodide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
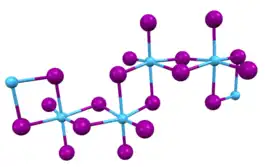
In this compound, the hafnium centers adopt octahedral coordination geometry. Like most binary metal halides, the compound is a polymeric. It is one-dimensional polymer consisting of chains of edge-shared bioctahedral Hf2I8 subunits, similar to the motif adopted by HfCl4. The nonbridging iodide ligands have shorter bonds to Hf than the bridging iodide ligands.[3]
References
- Haynes, William M., ed. (2011). CRC Handbook of Chemistry and Physics (92nd ed.). Boca Raton, FL: CRC Press. p. 4.66. ISBN 1-4398-5511-0.
- Krebs, B.; Sinram, D. (1980). "Hafniumtetrajodid HfI4: Struktur und eigenschaften. Ein neuer AB4-strukturtyp". Journal of the Less Common Metals. 76 (1–2): 7–16. doi:10.1016/0022-5088(80)90005-3.
- Krebs, B.; Sinram, D. (1980). "Hafniumtetrajodid HfI4: Struktur und eigenschaften. Ein neuer AB4-strukturtyp". Journal of the Less Common Metals. 76 (1–2): 7–16. doi:10.1016/0022-5088(80)90005-3.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.