Polonium tetraiodide
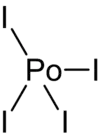
Polonium tetraiodide is a binary inorganic compound of polonium and iodine with the chemical formula PoI
4.[3][4] The compound forms volatile black crystals.[5]
 | |
| Names | |
|---|---|
| Other names
Polonium(IV) iodide | |
| Identifiers | |
3D model (JSmol) |
|
PubChem CID |
|
| |
| |
| Properties | |
| PoI 4[1] | |
| Molar mass | 716.6 g/mol |
| Appearance | Black crystals |
| Melting point | 200 °C (392 °F; 473 K) |
| Insoluble[2] | |
| Related compounds | |
Related compounds |
Chromium(III) sulfide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Synthesis
1. Action of iodine vapor on polonium metal:
2. Dissolution of polonium dioxide in hydroiodic acid:[6]
Properties
Physical properties
The compound forms black crystals that are insoluble in water.
Chemical properties
The compound reacts with hydroiodic acid to form hexaiodopolonic acid:
It can be reduced by hydrogen sulfide to yield polonium metal.[7] It decomposes on heating.
References
- Macintyre, Jane E. (23 July 1992). Dictionary of Inorganic Compounds. CRC Press. p. 3510. ISBN 978-0-412-30120-9. Retrieved 2 November 2021.
- Schweitzer, George K.; Pesterfield, Lester L. (14 January 2010). The Aqueous Chemistry of the Elements. Oxford University Press. p. 134. ISBN 978-0-19-539335-4. Retrieved 2 November 2021.
- Brown, Susan A.; Brown, Paul L. (25 September 2019). The Aqueous Chemistry of Polonium and the Practical Application of its Thermochemistry. Elsevier. p. 24. ISBN 978-0-12-819309-9. Retrieved 2 November 2021.
- Schmidt, M.; Siebert, W.; Bagnall, K.W. (2013). The Chemistry of Sulphur, Selenium, Tellurium and Polonium: Pergamon Texts in Inorganic Chemistry. Elsevier. pp. 961–962. ISBN 978-1483158655.
- Bagnall, K. W.; D'Eye, R. W. M.; Freeman, J. H. (1 January 1956). "657. The polonium halides. Part III. Polonium tetraiodide". Journal of the Chemical Society (Resumed): 3385–3389. doi:10.1039/jr9560003385. Retrieved 2 November 2021.
- M. Schmidt, W. Siebert, K. W. Bagnall (2013). The Chemistry of Sulphur, Selenium, Tellurium and Polonium: Pergamon Texts in Inorganic Chemistry. Elsevier. pp. 961–962. ISBN 978-1483158655.
{{cite book}}: CS1 maint: multiple names: authors list (link) - K. W. Bagnall, R. W. M. D'Eye, J. H. Freeman (1956). "657. The polonium halides. Part III. Polonium tetraiodide". Journal of the Chemical Society (Resumed). J. Chem. Soc.: 3385–3389. doi:10.1039/JR9560003385.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.