Thallium(I) iodide
Thallium(I) iodide is a chemical compound with the formula TlI. It is unusual in being one of the few water-insoluble metal iodides, along with AgI, CuI, SnI2, SnI4, PbI2 and HgI2.
 | |
| Names | |
|---|---|
| Other names
Thallium monoiodide Thallous iodide | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.029.272 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| TlI | |
| Molar mass | 331.287 g/mol[1] |
| Appearance | yellow crystals[1] |
| Density | 7.1 g/cm3[1] |
| Melting point | 441.7 °C (827.1 °F; 714.8 K)[1] |
| Boiling point | 824 °C (1,515 °F; 1,097 K)[1] |
| 0.085 g/L (25 °C)[1] | |
| Solubility | insoluble in alcohol[1] |
| −82.2·10−6 cm3/mol[2] | |
| Hazards | |
| GHS labelling: | |
   | |
| Danger | |
| H300, H330, H373, H411 | |
| P260, P264, P270, P271, P273, P284, P301+P310, P304+P340, P310, P314, P320, P321, P330, P391, P403+P233, P405, P501 | |
| Flash point | Non-flammable |
| Related compounds | |
Other anions |
Thallium(I) fluoride Thallium(I) chloride Thallium(I) bromide |
Other cations |
Gallium(I) iodide Indium(I) iodide |
Related compounds |
Mercury(II) iodide Lead(II) iodide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Chemistry
TlI can be formed in aqueous solution by metathesis of any soluble thallium salt with iodide ion. It is also formed as a by-product in thallium-promoted iodination of phenols with thallium(I) acetate.
Attempts to oxidise TlI to thallium(III) iodide fail, since oxidation produces thallium(I) triiodide, Tl+I3−.
Physical properties
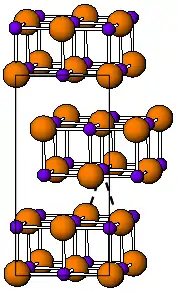
The room temperature form of TlI is yellow and has an orthorhombic structure [3] which can be considered to be a distorted NaCl structure. The distorted structure is believed to be caused by favourable thallium-thallium interactions, the closest Tl-Tl distance is 383 pm.[4] At 175 °C the yellow form transforms to a red CsCl form. This phase transition is accompanied by about two orders of magnitude jump in electrical conductivity. The CsI structure can be stabilized down to room temperature by doping TlI with other halides such as RbI, CsI, KI, AgI, TlBr and TlCl.[5] Thus, presence of impurities might be responsible for coexistence of the cubic and orthorhombic TlI phases at ambient conditions.[3] Under high pressure, 160 kbar, TlI becomes a metallic conductor. Nanometer-thin TlI films grown on LiF, NaCl or KBr substrates exhibit the cubic rocksalt structure.[6]
Applications
Thallium(I) iodide was initially added to mercury arc lamps to improve their performance[7] The light produced was mainly in the blue green part of the visible light spectrum least absorbed by water, so these have been used for underwater lighting.[8] In modern times, it is added to quartz and ceramic metal halide lamps that uses rare-earth halides like dysprosium, to increase their efficiency and to get the light color more close to the blackbody locus. Thallium iodide alone can be used to produces green colored metal halide lamps. Thallium(I) iodide is also used in trace amounts with NaI or CsI to produce scintillators used in radiation detectors.
Natural occurrence
Natural thallium(I) iodide was first discovered in a naturally occurring setting in 2017 as a orthorhombic polymorph called nataliyamalikite. Small grains were found embedded in mascagnite sourced from fumaroles at Avachinsky, a volcano in Russia's Kamchatka Peninsula that can reach temperatures of 640 °C (1,184 °F). The geologists that discovered it speculate that further research into this mineral is likely to add to the understanding of the geochemical evolution of the planet[9][10]
Safety
Like all thallium compounds, thallium(I) iodide is highly toxic.
References
- Haynes, p. 4.94
- Haynes, p. 4.136
- Lowndes, R. P.; Perry, C. H. (1973). "Molecular structure and anharmonicity in thallium iodide". The Journal of Chemical Physics. 58 (1): 271–278. Bibcode:1973JChPh..58..271L. doi:10.1063/1.1678917.
- Mudring, Anja-Verena (2007). "Thallium Halides – New Aspects of the Stereochemical Activity of Electron Lone Pairs of Heavier Main-Group Elements". European Journal of Inorganic Chemistry. 2007 (6): 882–890. doi:10.1002/ejic.200600975.
- Sultana, Saima; Rafiuddin (2009). "Electrical conductivity in TlI–TiO2 composite solid electrolyte". Physica B: Condensed Matter. 404 (1): 36–40. Bibcode:2009PhyB..404...36S. doi:10.1016/j.physb.2008.10.002.
- Schulz, L. G. (1951). "Polymorphism of cesium and thallium halides". Acta Crystallographica. 4 (6): 487–489. doi:10.1107/S0365110X51001641.
- Reiling, Gilbert H. (1964). "Characteristics of Mercury Vapor–Metallic Iodide Arc Lamps". Journal of the Optical Society of America. 54 (4): 532. Bibcode:1964JOSA...54..532R. doi:10.1364/JOSA.54.000532.
- Underwater Journal and information bulletin, IPC Science and Technology Press, (1973), p 245
- "Nataliyamalikite: Mineral information, data and localities". www.mindat.org.
- Anderson, Natali (July 6, 2017). "New Mineral Discovered: Nataliyamalikite". Sci News. Retrieved March 16, 2022.
Cited sources
- Haynes, William M., ed. (2011). CRC Handbook of Chemistry and Physics (92nd ed.). Boca Raton, FL: CRC Press. ISBN 1-4398-5511-0.