Antimony pentachloride
Antimony pentachloride is a chemical compound with the formula SbCl5. It is a colourless oil, but typical samples are yellowish due to dissolved chlorine. Owing to its tendency to hydrolyse to hydrochloric acid, SbCl5 is a highly corrosive substance and must be stored in glass or PTFE containers.
| |||
 | |||
| Names | |||
|---|---|---|---|
| IUPAC names
Antimony pentachloride Antimony(V) chloride | |||
| Other names
Antimonic chloride Antimony perchloride | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChemSpider | |||
| ECHA InfoCard | 100.028.729 | ||
| EC Number |
| ||
PubChem CID |
|||
| RTECS number |
| ||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| Cl5Sb | |||
| Molar mass | 299.01 g·mol−1 | ||
| Appearance | colorless or reddish-yellow (fuming) liquid, oily | ||
| Odor | pungent, offensive | ||
| Density | 2.336 g/cm3 (20 °C)[1] 2.36 g/cm3 (25 °C)[2] | ||
| Melting point | 2.8 °C (37.0 °F; 275.9 K) | ||
| Boiling point | 140 °C (284 °F; 413 K) decomposes from 106 °C[3] 79 °C (174 °F; 352 K) at 22 mmHg[1] 92 °C (198 °F; 365 K) at 30 mmHg[2] | ||
| reacts | |||
| Solubility | soluble in alcohol, HCl, tartaric acid, CHCl3, CS2, CCl4 | ||
| Solubility in selenium(IV) oxychloride | 62.97 g/100 g (25 °C) | ||
| Vapor pressure | 0.16 kPa (25 °C) 4 kPa (40 °C) 7.7 kPa (100 °C)[4] | ||
| -120.0·10−6 cm3/mol | |||
Refractive index (nD) |
1.59255 | ||
| Viscosity | 2.034 cP (29.4 °C)[1] 1.91 cP (35 °C) | ||
| Structure | |||
| Trigonal bipyramidal | |||
| 0 D | |||
| Thermochemistry[3] | |||
Heat capacity (C) |
120.9 J/mol·K (gas) | ||
Std molar entropy (S⦵298) |
295 J/mol·K | ||
Std enthalpy of formation (ΔfH⦵298) |
-437.2 kJ/mol | ||
Gibbs free energy (ΔfG⦵) |
-345.35 kJ/mol | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Inhalation hazards |
Toxic | ||
| GHS labelling:[2] | |||
  | |||
| Danger | |||
| H314, H411 | |||
| P273, P280, P305+P351+P338, P310 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 77 °C (171 °F; 350 K) | ||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose) |
1115 mg/kg, (rat, oral)[3] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible) |
TWA 0.5 mg/m3 (as Sb)[5] | ||
REL (Recommended) |
TWA 0.5 mg/m3 (as Sb)[5] | ||
| Related compounds | |||
Other anions |
Antimony pentafluoride | ||
Other cations |
Phosphorus pentachloride | ||
Related compounds |
Antimony trichloride | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |||
Preparation and structure
Antimony pentachloride is prepared by passing chlorine gas into molten antimony trichloride:
- SbCl3 + Cl2 → SbCl5
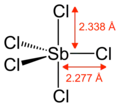
Gaseous SbCl5 has a trigonal bipyramidal structure.[6]
Reactions
This compounds reacts with water to form antimony pentoxide and hydrochloric acid:[7]
- 2 SbCl5 + 5 H2O → Sb2O5 + 10 HCl
The mono- and tetrahydrates are known, SbCl5·H2O and SbCl5·4H2O.
This compound forms adducts with many Lewis bases. SbCl5 is a soft Lewis acid and its ECW model parameters are EA = 3.64 and CA = 10.42. It is used as the standard Lewis acid in the Gutmann scale of Lewis basicity.[8][9]
It is also a strong oxidizing agent.[10] For example aromatic ethers are oxidized to their radical cations according to the following stoichiometry:[11]
- 3 SbCl5 + 2 ArH → 2 (ArH+)(SbCl6−) + SbCl3
Applications
Antimony pentachloride is used as a polymerization catalyst and for the chlorination of organic compounds.
Precautions
Antimony pentachloride is a highly corrosive substance that should be stored away from heat and moisture. It is a chlorinating agent and, in the presence of moisture, it releases hydrogen chloride gas. Because of this, it may etch even stainless-steel tools (such as needles), if handled in a moist atmosphere. It should not be handled with non-fluorinated plastics (such as plastic syringes, plastic septa, or needles with plastic fittings), since it melts and carbonizes plastic materials.[12]
References
- "Antimony pentachloride (UK PID)".
- Sigma-Aldrich Co., Antimony(V) chloride. Retrieved on 2014-05-29.
- "Antimony(V) chloride".
- Antimony pentachloride in Linstrom, Peter J.; Mallard, William G. (eds.); NIST Chemistry WebBook, NIST Standard Reference Database Number 69, National Institute of Standards and Technology, Gaithersburg (MD) (retrieved 2014-05-29)
- NIOSH Pocket Guide to Chemical Hazards. "#0036". National Institute for Occupational Safety and Health (NIOSH).
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- Pradyot Patnaik. Handbook of Inorganic Chemicals. McGraw-Hill, 2002, ISBN 0-07-049439-8
- V. Gutmann (1976). "Solvent effects on the reactivities of organometallic compounds". Coord. Chem. Rev. 18 (2): 225–255. doi:10.1016/S0010-8545(00)82045-7.
- Cramer, R. E.; Bopp, T. T. (1977). "Graphical display of the enthalpies of adduct formation for Lewis acids and bases". Journal of Chemical Education. 54: 612–613. doi:10.1021/ed054p612. The plots shown in this paper used older parameters. Improved E&C parameters are listed in ECW model.
- Connelly, N. G.; Geiger, W. E. (1996). "Chemical Redox Agents for Organometallic Chemistry". Chem. Rev. 96 (2): 877–922. doi:10.1021/cr940053x. PMID 11848774.
- Rathore, R.; Kumar, A. S.; Lindeman, S. V.; Kochi, J. K. (1998). "Preparation and Structures of Crystalline Aromatic Cation-Radical Salts. Triethyloxonium Hexachloroantimonate as a Novel (One-Electron) Oxidant". The Journal of Organic Chemistry. 63 (17): 5847–5856. doi:10.1021/jo980407a. PMID 11672186.
- Shekarchi, M.; Behbahani, F. K Catal. Lett. 2017 147 2950. doi:10.1007/s10562-017-2194-2