Kinyoun stain
The Kinyoun method or Kinyoun stain (cold method), developed by Joseph J. Kinyoun, is a procedure used to stain acid-fast species of the bacterial genus Mycobacterium.[1] It is a variation of a method developed by Robert Koch in 1882. Certain species of bacteria have a waxy lipid called mycolic acid, in their cell walls which allow them to be stained with Acid-Fast better than a Gram-Stain. The unique ability of mycobacteria to resist decolorization by acid-alcohol is why they are termed acid-fast.[2] It involves the application of a primary stain (basic fuchsin), a decolorizer (acid-alcohol), and a counterstain (methylene blue).[3] Unlike the Ziehl–Neelsen stain (Z-N stain), the Kinyoun method of staining does not require heating.[4][5] In the Ziehl–Neelsen stain, heat acts as a physical mordant while phenol (carbol of carbol fuchsin) acts as the chemical mordant. Since the Kinyoun stain is a cold method (no heat applied), the concentration of carbol fuschin used is increased.[6]
| Application of | Reagent | Cell colour | |
|---|---|---|---|
| Acid fast | Non-acid fast | ||
| Primary dye | Carbol fuchsin | Red | Red |
| Decolorizer | Acid alcohol | Red | Colorless |
| Counter Stain | Methylene blue | Red | Blue |
Staining procedure
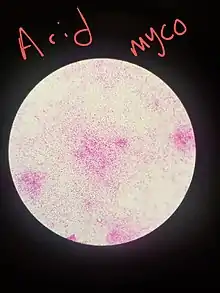
Materials
- Slide with organism smear
- Carbol Fuchsin
- Acid-Alcohol
- Methylene blue
- bibulous paper
- Microscope
Instructions
- Make smear on a slide with organisms
- Clean slide, wax label slide, spread organism, air dry for 10 minutes, heat fix
- Dip slide into Carbol Fuchsin for 20 minutes.
- Rinse slide
- Dip slide into Acid-alcohol for 3–5 seconds.
- Rinse Slide
- Dip slide into Methylene blue for 30 seconds.
- Rinse Slide
- Blot slide dry with bibulous paper.
- Observe under Microscope.
Modification
The Kinyoun method can be modified as a weak acid fast stain, which uses 0.5–1.0% sulfuric acid instead of hydrochloric acid. The weak acid fast stain, in addition to staining Mycobacteria, will also stain organisms that are not able to maintain the carbol fuchsin after decolorizing with HCl, such as Nocardia species and Cryptosporidium.
See also
References
- Geo F. B et al, Jawetz, Melnick and Adelberg's Medical Microbiology, 25th Edition, Lange Medical,2004, page 182
- .dalynn (October 2014). "KINYOUN CARBOL FUCHSIN STAIN" (PDF). Archived (PDF) from the original on 2022-01-14. Retrieved 2019-05-22.
- Hussey, A. M., Zayaitz, A., "Acid-Fast Stain Protocols" Archived 2011-10-01 at the Wayback Machine, American Society for Microbiology, 8 September 2008. Retrieved on 1 November 2014.
- Murray PR, Baron E, Pfaller M, Tenover F, Yolken, Eds. Manual of clinical microbiology. 7th ed. Washington, DC: ASM, 1999.
- Baron EJ, Finegold SM. Bailey and Scott's diagnostic microbiology. 8th ed. St. Louis: Mosby, 1990.
- Ananthanarayan and Panicker's Textbook of Microbiology, 9th Edition, Universities Press (India), 2013, page 353
- Briggs, Chris (July 2018). "Part 3: Differential and Structural Staining". Microbiology Lab Manual: Discovering Your Tiny Neighbors (PDF). p. 58. Archived from the original (PDF) on 2022-01-14.
{{cite book}}:|work=ignored (help)