Lead(IV) sulfide
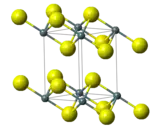
Lead(IV) sulfide is a chemical compound with the formula PbS2. This material is generated by the reaction of the more common lead(II) sulfide, PbS, with sulfur at >600 °C and at high pressures. PbS2, like the related tin(IV) sulfide SnS2, crystallises in the cadmium iodide motif, which indicates that Pb should be assigned the formal oxidation state of 4+.[1]
 | |
| Names | |
|---|---|
| IUPAC name
lead(IV) sulfide | |
| Other names
lead disulfide | |
| Identifiers | |
3D model (JSmol) |
|
| ECHA InfoCard | 100.032.025 |
PubChem CID |
|
| |
| |
| Properties | |
| PbS2 | |
| Molar mass | 271.332 g/mol |
| Structure[1] | |
| Rhombohedral, hP3 | |
| P3m1, No. 164 | |
| 3 2/m | |
a = 3.89 Å, c = 5.91 Å α = 90°, β = 90°, γ = 120° | |
| Octahedral (Pb4+) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Lead(IV) sulfide is a p-type semiconductor, and is also a thermoelectric material.[2]
References
- Silverman, M. S. (1966). "High-pressure (70-kilobar) Synthesis of New Crystalline Lead Dichalcogenides". Inorganic Chemistry. 5 (11): 2067–9. doi:10.1021/ic50045a056.
- Cava, R.J. (2011). "Pressure Stabilized Se-Se Dimer Formation in PbSe2". Solid State Sciences. 13: 38–41. doi:10.1016/j.solidstatesciences.2010.10.003.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.