Zirconium disulfide
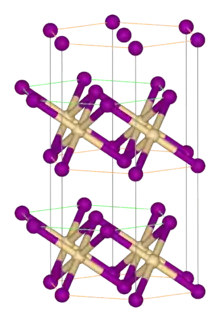
Zirconium(IV) sulfide is the inorganic compound with the formula ZrS2. It is a violet-brown solid. It adopts a layered structure similar to that of cadmium iodide.
 | |
| Names | |
|---|---|
| IUPAC name
Zirconium(IV) sulfide | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.031.701 |
| EC Number |
|
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| ZrS2 | |
| Molar mass | 155.356 g/mol |
| Appearance | red-brown crystals |
| Density | 3.82 g/cm3 |
| Melting point | 1,480 °C (2,700 °F; 1,750 K) |
| insoluble | |
| Structure | |
| Rhombohedral, hP3 | |
| P-3m1, No. 164 | |
| octahedral | |
| Hazards | |
| Safety data sheet (SDS) | MSDS |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Like the closely related titanium disulfide, ZrS2 is prepared by heating sulfur and zirconium metal. It can be purified by vapor transport using iodine.[2]
References
- Lide, David R. (1998), Handbook of Chemistry and Physics (87 ed.), Boca Raton, Florida: CRC Press, pp. 4–96, ISBN 0-8493-0594-2
- Lawrence E. Conroy "Group IV Sulfides" Inorganic Synthesis 1970, XII, 158. doi:10.1002/9780470132432.ch28
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.