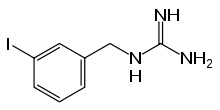
Iobenguane
Iobenguane, or MIBG, is an aralkylguanidine analog of the adrenergic neurotransmitter norepinephrine (noradrenaline), typically used as a radiopharmaceutical.[3] It acts as a blocking agent for adrenergic neurons. When radiolabeled, it can be used in nuclear medicinal diagnostic and therapy techniques as well as in neuroendocrine chemotherapy treatments.
 | |
| Clinical data | |
|---|---|
| Trade names | Adreview, Azedra |
| Other names | meta-iodobenzylguanidine mIBG, MIBG |
| License data |
|
| Routes of administration | Intravenous |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C8H10IN3 |
| Molar mass | 275.093 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
It localizes to adrenergic tissue and thus can be used to identify the location of tumors such as pheochromocytomas and neuroblastomas.[4] With iodine-131 it can also be used to treat tumor cells that take up and metabolize norepinephrine.
Usage and mechanism
MIBG is absorbed by and accumulated in granules of adrenal medullary chromaffin cells, as well as in pre-synaptic adrenergic neuron granules. The process in which this occurs is closely related to the mechanism employed by norepinephrine and its transporter in vivo.[5][6] The norepinephrine transporter (NET) functions to provide norepinephrine uptake at the synaptic terminals and adrenal chromaffin cells. MIBG, by bonding to NET, finds its roles in imaging and therapy.
Metabolites and excretion
Less than 10% of the administered MIBG gets metabolized into m-iodohippuric acid (MIHA), and the mechanism for how this metabolite is produced is unknown.[7]
Diagnostic imaging
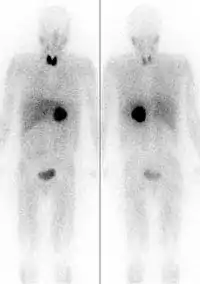
MIBG concentrates in endocrine tumors, most commonly neuroblastoma, paraganglioma, and pheochromocytoma. It also accumulates in norepinephrine transporters in adrenergic nerves in the heart, lungs, adrenal medulla, salivary glands, liver, and spleen, as well as in tumors that originate in the neural crest. When labelled with iodine-123 it serves as a whole-body, non-invasive scintigraphic screening for germ-line, somatic, benign, and malignant neoplasms originating in the adrenal glands. It can detect both intra and extra-adrenal disease. The imaging is highly sensitive and specific.[8][9]
Iobenguane concentrates in presynaptic terminals of the heart and other autonomically innervated organs. This enables the possible non-invasive use as an in vivo probe to study these systems.[10][11]
Alternatives to imaging with 123I-MIBG, for certain indications and under clinical and research use, include the positron-emitting isotope iodine-124, and other radiopharmaceuticals such as 68Ga-DOTA and 18F-FDOPA for positron emission tomography (PET).[9][12][13] 123I-MIBG imaging on a gamma camera can offer significantly higher cost-effectiveness and availability compared to PET imaging, and is particularly effective where 131I-MIBG therapy is subsequently planned, due to their directly comparable uptake.[14][9][15]
Side effects
Side effects post imaging are rare but can include tachycardia, pallor, vomiting, and abdominal pain.[9]
Radionuclide therapy
MIBG can be radiolabelled with the beta emitting radionuclide 131I for the treatment of certain pheochromocytomas, paragangliomas, carcinoid tumors, neuroblastomas, and medullary thyroid cancer.[16]
Thyroid precautions
Thyroid blockade with (nonradioactive) potassium iodide is indicated for nuclear medicine scintigraphy with iobenguane/mIBG. This competitively inhibits radioiodine uptake, preventing excessive radioiodine levels in the thyroid and minimizing risk of thyroid ablation (in treatment with 131I). The minimal risk of thyroid cancer is also reduced as a result.[9][17]
The dosing regime for the FDA-approved commercial 123I-MIBG product Adreview is potassium iodide or Lugol's solution containing 100 mg iodide, weight adjusted for children and given an hour before injection.[18] EANM guidelines, endorsed by the SNMMI, suggest a variety of regimes in clinical use, for both children and adults.[9][12]
Product labeling for diagnostic 131I iobenguane recommends giving potassium iodide one day before injection and continuing 5 to 7 days following.[19] 131I iobenguane used for therapeutic purposes requires a different pre-medication duration, beginning 24–48 hours before iobenguane injection and continuing 10–15 days after injection.[16]
Clinical trials
Iobenguane I 131 for cancers
Iobenguane I 131, marketed under the trade name Azedra, has had a clinical trial as a treatment for malignant, recurrent or unresectable pheochromocytoma and paraganglioma, and the FDA approved it on July 30, 2018. The drug is developed by Progenics Pharmaceuticals.[20][21]
References
- "Adreview- iobenguane i-123 injection". DailyMed. 10 March 2020. Retrieved 21 May 2022.
- "Azedra- iobenguane i-131 injection, solution". DailyMed. 8 April 2021. Retrieved 21 May 2022.
- Olecki E, Grant CN (December 2019). "MIBG in neuroblastoma diagnosis and treatment". Seminars in Pediatric Surgery. 28 (6): 150859. doi:10.1016/j.sempedsurg.2019.150859. PMID 31931960. S2CID 210191760.
- Scarsbrook AF, Ganeshan A, Statham J, Thakker RV, Weaver A, Talbot D, et al. (2007). "Anatomic and functional imaging of metastatic carcinoid tumors". Radiographics. 27 (2): 455–77. doi:10.1148/rg.272065058. PMID 17374863.
- Beylergil V, Perez JA, Osborne JR (2016). "Molecular Imaging of Merkel Cell Carcinoma". In Hamblin MR, Avci P, Gupta GK (eds.). Imaging in dermatology. London. pp. 467–470. doi:10.1016/B978-0-12-802838-4.00033-9. ISBN 978-0-12-802838-4.
Metaiodobenzylguanidine (MIBG) is a radiolabeled analogue of guanethidine that enters the cells via the norepinephrine transporter and is either stored in the cytoplasm or in secretory granules
{{cite book}}: CS1 maint: location missing publisher (link) - Davidoff AM (2010). "Neuroblastoma". In Holcomb GW, Murphy JP, Ostlie DJ (eds.). Ashcraft's pediatric surgery (5th ed.). Philadelphia: Saunders/Elsevier. pp. 872–894. doi:10.1016/B978-1-4160-6127-4.00068-9. ISBN 978-1-4160-6127-4.
Metaiodobenzylguanidine (MIBG) is transported to and stored in the distal storage granules of chromaffin cells in the same way as norepinephrine.
- "Iobenguane". DrugBank Online. Retrieved 19 August 2021. from Wishart DS, Feunang YD, Guo AC, Lo EJ, Marcu A, Grant JR, et al. (January 2018). "DrugBank 5.0: a major update to the DrugBank database for 2018". Nucleic Acids Research. 46 (D1): D1074–D1082. doi:10.1093/nar/gkx1037. PMC 5753335. PMID 29126136.
- Vlachou FJ (2018). "SPECT in Adrenal Glands". In Gouliamos AD, Andreou JA, Kosmidis PA (eds.). Imaging in Clinical Oncology. Cham: Springer. p. 481. doi:10.1007/978-3-319-68873-2_70. ISBN 978-3-319-68872-5. S2CID 52922868.
- Taïeb D, Hicks RJ, Hindié E, Guillet BA, Avram A, Ghedini P, et al. (September 2019). "European Association of Nuclear Medicine Practice Guideline/Society of Nuclear Medicine and Molecular Imaging Procedure Standard 2019 for radionuclide imaging of phaeochromocytoma and paraganglioma". European Journal of Nuclear Medicine and Molecular Imaging. 46 (10): 2112–2137. doi:10.1007/s00259-019-04398-1. PMC 7446938. PMID 31254038.
- Das S, Gordián-Vélez WJ, Ledebur HC, Mourkioti F, Rompolas P, Chen HI, et al. (December 2020). "Innervation: the missing link for biofabricated tissues and organs". npj Regenerative Medicine. 5 (1): 11. doi:10.1038/s41536-020-0096-1. PMC 7275031. PMID 32550009.
- Sisson JC, Shapiro B, Meyers L, Mallette S, Mangner TJ, Wieland DM, et al. (October 1987). "Metaiodobenzylguanidine to map scintigraphically the adrenergic nervous system in man". Journal of Nuclear Medicine. 28 (10): 1625–36. PMID 3655915.
- Bar-Sever Z, Biassoni L, Shulkin B, Kong G, Hofman MS, Lopci E, et al. (October 2018). "Guidelines on nuclear medicine imaging in neuroblastoma". European Journal of Nuclear Medicine and Molecular Imaging. 45 (11): 2009–2024. doi:10.1007/s00259-018-4070-8. PMID 29938300. S2CID 49410438.
- Rufini V, Treglia G, Castaldi P, Perotti G, Giordano A (June 2013). "Comparison of metaiodobenzylguanidine scintigraphy with positron emission tomography in the diagnostic work-up of pheochromocytoma and paraganglioma: a systematic review". The Quarterly Journal of Nuclear Medicine and Molecular Imaging. 57 (2): 122–33. PMID 23822989.
- Čtvrtlík F, Koranda P, Schovánek J, Škarda J, Hartmann I, Tüdös Z (April 2018). "Current diagnostic imaging of pheochromocytomas and implications for therapeutic strategy". Experimental and Therapeutic Medicine. 15 (4): 3151–3160. doi:10.3892/etm.2018.5871. PMC 5840941. PMID 29545830.
- Ballinger JR (November 2018). "Theranostic radiopharmaceuticals: established agents in current use". The British Journal of Radiology. 91 (1091): 20170969. doi:10.1259/bjr.20170969. PMC 6475961. PMID 29474096.
- Giammarile F, Chiti A, Lassmann M, Brans B, Flux G (May 2008). "EANM procedure guidelines for 131I-meta-iodobenzylguanidine (131I-mIBG) therapy". European Journal of Nuclear Medicine and Molecular Imaging. 35 (5): 1039–47. doi:10.1007/s00259-008-0715-3. PMID 18274745. S2CID 6884201.
- Van Vickle SS, Thompson RC (June 2015). "123I-MIBG Imaging: Patient Preparation and Technologist's Role". Journal of Nuclear Medicine Technology. 43 (2): 82–6. doi:10.2967/jnmt.115.158394. PMID 25956690.
- "Drug Approval Package: AdreView (Iobenguane I 123) NDA # 022290". U.S. Food and Drug Administration. 2008. Retrieved 19 August 2021.
- "Iobenguane Sulfate I 131 Injection Diagnostic package insert". Bedford, MA: CIS-US, Inc. July 1999 – via Drugs.com.
- "AZEDRA® (iobenguane I 131". Billerica, MA: Progenics Pharmaceuticals Inc., a Lantheus company. July 2018.
- "FDA approves first treatment for rare adrenal tumors". U.S. Food and Drug Administration. 30 July 2018.
External links
- "Iobenguane". Drug Information Portal. U.S. National Library of Medicine.
- "Iobenguane I 131". Drug Information Portal. U.S. National Library of Medicine.
- "Iobenguane I 123". Drug Information Portal. U.S. National Library of Medicine.
- "iobenguane I 131". NCI Drug Dictionary.
- "Iobenguane I 131". National Cancer Institute. 15 August 2018.