Marmesin
Marmesin (nodakenetin) is a chemical compound precursor in psoralen and linear furanocoumarins biosynthesis.[1]
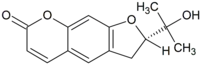 | |
| Names | |
|---|---|
| Preferred IUPAC name
(2S)-2-(2-Hydroxypropan-2-yl)-2,3-dihydro-7H-furo[3,2-g][1]benzopyran-7-one | |
| Other names
Nodakenetin | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C14H14O4 | |
| Molar mass | 246.262 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Marmesin plays a central role in the biosynthesis of furocoumarins in the plant ruta graveolens, more commonly known as rue. It acts as the natural intermediate in the formation of the furan ring that leads to a 4’,5’-dihydro furocoumarin-derivative. This substance can then be transformed into psoralen and other furocoumarins present in rue. Upon feeding the herb a dose of marmesin, radioactivity became strongly incorporated into psoralen and thus the plant itself.[2]
Spectra
IR Spectra
IR (ATR): νmax 3480, 2971, 1699, 1631, 1488 cm-1.[3]
Proton-NMR
1H-NMR (300 MHz, CDCl3): δ 7.59 (d, J = 9.5 Hz, 1H, aromatic), 7.22 (s, 1H, aromatic), 6.75 (d, J = 21.6 Hz, 1H, aromatic), 6.20 (d, J = 9.5 Hz, 1H, aromatic), 4.74 (t, J = 8.8 Hz, 1H, CH), 3.28-3.15 (m, 2H, CH2), 1.87 (s, 1H, OH), 1.37 (s, 3H, CH3), 1.24 (s, 3H, CH3) ppm.[4]
UV-Vis
UV: [neutral]λmax 217 (ε7420); 338 (ε17700)( MeOH) [neutral]λmax 332( EtOH).[5]
Production
Synthesis of marmesin has been successfully conducted in the laboratory on multiple occasions. One way of doing so is by a strategy based on the palladium-catalyzed intramolecular coupling reaction. This reaction would construct the dihydropyran ring and synthesize the compound from the intermediate (-)-peucedanol. The key step in the overall synthesis uses catalytic asymmetric epoxidation of an enone.[6]
References
- Steck, Warren; Brown, Stewart A. (1971). "Comparison of (+)- and (−)-Marmesin as Intermediates in the Biosynthesis of Linear Furanoconmarins". Biochemistry and Cell Biology. 49 (11): 1213–1216. doi:10.1139/o71-174. ISSN 1208-6002. PMID 5134594.
- Caporale, G.; Dall’Acqua, F.; Capozzi, A.; Marciani, S.; Crocco, R. Studies on the biosynthesis of some furocoumarins present in Ruta graveolens. Zeitschrift für Naturforschung B 1971, 26, 1256-1259.
- Ando, T.; Nagumo, M.; Ninomiya, M.; Tanaka, K.; Linhardt, R. J.; Koketsu, M. Synthesis of coumarin derivatives and their cytoprotective effects on t-BHP-induced oxidative damage in HepG2 cells. Bioorg. Med. Chem. Lett. 2018, 28, 2422-2425.
- Kommera, R.; Bhimapaka, C. R. A simple and efficient approach for the preparation of dihydroxanthyletin, xanthyletin, decursinol and marmesin. Synthetic Communications 2020, 50, 3204-3211.
- Anonymous Dictionary of natural products; Chapman & Hall: London, 1994.
- Nemoto, T.; Ohshima, T.; Shibasaki, M. Enantioselective total syntheses of novel PKC activator (+)-decursin and its derivatives using catalytic asymmetric epoxidation of an enone. Tetrahedron Lett. 2000, 41, 9569-9574.
External links
- Abu-Mustafa, Effat A.; Fayez, M. B. E. (1961). "Natural Coumarins. I. Marmesin and Marmesinin, Further Products from the Fruits of Ammi majus L.". The Journal of Organic Chemistry. 26 (1): 161–166. doi:10.1021/jo01060a039. ISSN 0022-3263.