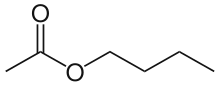
Butyl acetate
n-Butyl acetate is an organic compound with the formula CH3CO2(CH2)3CH3. A colorless, flammable liquid, it is the ester derived from n-butanol and acetic acid. It is found in many types of fruit, where it imparts characteristic flavors and has a sweet smell of banana or apple. It is used as an industrial solvent.[7]
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Butyl acetate | |
| Systematic IUPAC name
Butyl ethanoate | |
| Other names
n-Butyl acetate Acetic acid n-butyl ester Butile | |
| Identifiers | |
3D model (JSmol) |
|
| Abbreviations | BuAcO |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.004.236 |
| EC Number |
|
| KEGG | |
PubChem CID |
|
| RTECS number |
|
| UNII | |
| UN number | 1123 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| CH3CO2(CH2)3CH3 | |
| Molar mass | 116.160 g·mol−1 |
| Appearance | Colorless liquid |
| Odor | Fruity |
| Density | 0.8825 g/cm3 (20 °C)[1] |
| Melting point | −78 °C (−108 °F; 195 K)[1] |
| Boiling point | 126.1 °C (259.0 °F; 399.2 K) at 760 mmHg[1] |
| 0.68 g/100 mL (20 °C)[1] | |
| Solubility | Miscible in EtOH Soluble in acetone, CHCl3[1] |
| log P | 1.82[1] |
| Vapor pressure | |
Henry's law constant (kH) |
0.281 L·atm/mol |
| −77.47·10−6 cm3/mol | |
| Thermal conductivity |
|
Refractive index (nD) |
1.3941 (20 °C)[1] |
| Viscosity |
|
| Structure | |
| 1.87 D (24 °C)[1] | |
| Thermochemistry | |
Heat capacity (C) |
225.11 J/mol·K[2] |
Std enthalpy of formation (ΔfH⦵298) |
−609.6 kJ/mol[2] |
Std enthalpy of combustion (ΔcH⦵298) |
3467 kJ/mol[2] |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards |
Flammable |
| GHS labelling: | |
  [3] [3] | |
| Warning | |
| H226, H336[3] | |
| P261[3] | |
| NFPA 704 (fire diamond) | |
| Flash point | 22 °C (72 °F; 295 K)[4] |
| 370 °C (698 °F; 643 K)[4] | |
Threshold limit value (TLV) |
150 ppm[1] (TWA), 200 ppm[1] (STEL) |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
10768 mg/kg (rats, oral)[4] |
LC50 (median concentration) |
160 ppm (rat, 4 hr) 2000 ppm (rat, 4 hr) 391 ppm (rat, 4 hr) 1242 ppm (mouse, 2 hr)[5] |
LCLo (lowest published) |
14,079 ppm (cat, 72 min) 13,872 ppm (guinea pig, 4 hr)[5] |
| NIOSH (US health exposure limits): | |
PEL (Permissible) |
TWA 150 ppm (710 mg/m3)[4] |
REL (Recommended) |
TWA 150 ppm (710 mg/m3) ST 200 ppm (950 mg/m3)[6] |
IDLH (Immediate danger) |
1700 ppm[6] |
| Related compounds | |
Related acetates |
Ethyl acetate Propyl acetate Amyl acetate |
Related compounds |
Butanol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
The other three isomers (four, including stereoisomers) of butyl acetate are isobutyl acetate, tert-butyl acetate, and sec-butyl acetate (two enantiomers).
Production and use
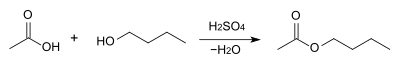
Butyl acetate is commonly manufactured by the Fischer esterification of butanol (or its isomer to make an isomer of butyl acetate) and acetic acid with the presence of sulfuric acid:[7]
Butyl acetate is mainly used as a solvent for coatings and inks.[7] It is a component of fingernail polish.[8]
Occurrence in nature
Apples, especially of the 'Red Delicious' variety, are flavored in part by this chemical. The alarm pheromones emitted by the Koschevnikov gland of honey bees contain butyl acetate.
References
- Lide, David R., ed. (2009). CRC Handbook of Chemistry and Physics (90th ed.). Boca Raton, Florida: CRC Press. ISBN 978-1-4200-9084-0.
- Acetic acid, butyl ester in Linstrom, Peter J.; Mallard, William G. (eds.); NIST Chemistry WebBook, NIST Standard Reference Database Number 69, National Institute of Standards and Technology, Gaithersburg (MD) (retrieved 2014-06-28)
- Sigma-Aldrich Co., Butyl acetate. Retrieved on 2014-06-28.
- "MSDS of n-Butyl acetate". fishersci.ca. Fisher Scientific. Retrieved 2014-06-28.
- "n-Butyl acetate". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- NIOSH Pocket Guide to Chemical Hazards. "#0072". National Institute for Occupational Safety and Health (NIOSH).
- Cheung, Hosea; Tanke, Robin S.; Torrence, G. Paul (2000). "Acetic Acid". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a01_045.
- Schneider, Günther; Gohla, Sven; Schreiber, Jörg; Kaden, Waltraud; Schönrock, Uwe; Schmidt-Lewerkühne, Hartmut; Kuschel, Annegret; Petsitis, Xenia; Pape, Wolfgang; Ippen, Hellmut; Diembeck, Walter (2001). "Skin Cosmetics". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a24_219. ISBN 3527306730.