Pentyl butyrate
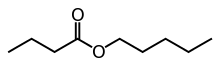
Pentyl butyrate, also known as pentyl butanoate or amyl butyrate, is an ester that is formed when pentanol is reacted with butyric acid,[1] usually in the presence of sulfuric acid as a catalyst. This ester has a smell reminiscent of pear or apricot. This chemical is used as an additive in cigarettes.
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Pentyl butanoate | |
| Other names
Pentyl butyrate | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.007.946 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C9H18O2 | |
| Molar mass | 158.24 g/mol |
| Odor | Apricot |
| Density | 0.86 g/cm3 |
| Melting point | −73.2 °C (−99.8 °F; 200.0 K) |
| Boiling point | 186 °C (367 °F; 459 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
References
- N-AMYL BUTYRATE, Cameo Chemicals, National Oceanic and Atmospheric Administration
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.