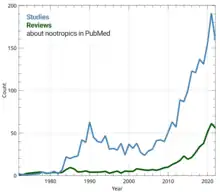
Neuroenhancement
Neuroenhancement or cognitive enhancement refers to the targeted enhancement and extension of cognitive and affective abilities based on an understanding of their underlying neurobiology in healthy persons who do not have any mental illness and outcomes in experimental research.[1][2][3][4][5][6][7] As such, it can be thought of as an umbrella term that encompasses pharmacological and non-pharmacological methods of improving neurological functionality, especially interventions designed to improve human form or functioning beyond what is necessary to sustain or restore good health, as well as the overarching ethico-legal discourse that accompanies these aims and practices.[8][9]
Neuroenhancers reliably engender substantial cognitive, social, psychological, mood, or motor benefits beyond normal functioning in healthy individuals,[9][7] whilst causing few side effects, albeit broader definitions also include the use of psychoactive substances that are deemed unhealthy or have substantial side effects. Pharmacological neuroenhancement agents include well-validated nootropics, such as modafinil,[13] Bacopa monnieri,[19] phosphatidylserine,[6] and caffeine,[27] as well as other drugs used for treating patients with neurological disorders.
Non-pharmacological measures of cognitive enhancement include behavioral methods (activities, techniques, and changes),[28] non-invasive brain stimulation, which has been employed to improve various cognitive and affective functions, and brain-machine interfaces, which hold much potential to extend the repertoire of motor and cognitive capacities.[29]
Pharmacological
There are many nootropics, which include smart drugs and dietary supplements, and all or many of these are relevant to neuroenhancement, albeit many or most only have small effect sizes in healthy individuals or common major side effects. The most common, popular[31][32] or notable[33] pharmacological agents in neuroenhancement with potentials for significant effect sizes (as in at least as effective[34][12] or similar to caffeine)[9] include modafinil and methylphenidate (Ritalin).
Stimulants in general[20][22] and various antidementives,[20][22][35][36] anxiolytics,[35] empathogens,[37] types of microdosing (mainly of psychedelics),[37][38][39][40] and antidepressants[20][22] may also fall into the scope of neuroenhancement despite not necessarily being considered nootropics.
Although consideration of individual neuroenhancement agents is usually triggered by success in clinical and technological fields, they have also been used to attempt to help people with a lack of normal cognitive, motor, and affective abilities: for example, social skills and empathy. In this case, neuroenhancement drugs try to increase oxytocin and decrease cortisol levels helping people better their communication and social interaction skills.[5][41]
Neuroenhancement is not only concerned with, short- and longer-term, enhancement of intelligence (by various types of measures), learning (e.g. general memory enhancement), focus/flow-state,[9][42][43][44][41] and related cognitive domains or measures but also:
- mood ('mood enhancement')[25][45][36][41]
- motivation[42][38][39][43] ('motivation enhancement'; e.g. apathy-related, in goal-setting, will to power, anhedonia or for exercise[46])
- task-enjoyment[47]
- sociability (e.g. talking-related or empathy)[48][49][50][51][37][41]
- substituting and/or ending unhealthy substances use (substance use disorders[52][53][54][55] or low/moderate consumption)
- discipline or will-power and self-control[56]
- creativity[38][39][43][57][58]
- cognitive endurance[59] (cognitive work endurance[42][47][43] and fatigue resistance)[60]
- specific capacities and skills such as verbal fluency, types of problem-solving, reasoning, simple and complex reaction time, and so on[61][12][62][57]
- psychological resilience[63][64]
- and more[35]
Enhancers are multidimensional[33] and can be clustered into biochemical, physical, and behavioral enhancement strategies.[33] N-acetylcysteine (NAC) is an example of a low side effects cognitive enhancer relevant to both unhealthy substance use change[52][53][65] and mood stabilization.[66][67][68][69][70]
Modafinil
Modafinil is a wakefulness-promoting drug that decreases fatigue, increases vigilance, reduces daytime sleepiness, and improves mood.[4][5][10] Modafinil is currently licensed for treating patients with disorders such as narcolepsy, sleep apnea, and shift work sleep disorder.[2][5] This drug also seems promising in the treatment of depression and bipolar disorder.[5] Modafinil is currently being used by United States Air Force personnel for missions of great duration in an attempt to decrease fatigue amongst aircrew. It has become more popular amongst the general public. In an online poll conducted by Nature magazine, 8.8% of 1400 corresponding readers admitted use of modafinil for non-medical reasons. Their reasoning behind its use was for increasing concentration and focus on a specific task or to counteract sleep deficit and jetlag.[2] A comparison between the sales of modafinil to the number of patients revealed a disproportionate ratio, indicating high abuse.[2]
Modafinil has been reported to improve executive function in healthy non-sleep-deprived individuals, as well as potentially improving attention and learning and memory.[1] Effects on sleep-deprived individuals are even more striking: a single dose resulted in enhanced wakefulness, executive functions, and memory.[10] In the case of sustained sleep deprivation, repeated intake of modafinil helped individuals maintain higher levels of wakefulness than the placebo, but did not help attention and executive function.[2][10] Since the majority of these trials were conducted on military personnel, further research needs to be conducted on the effects of modafinil on the general population. Modafinil may impair one's self-monitoring ability. A common trend found in research studies indicated that participants rated their performances on cognitive tests higher than it was, suggesting an "overconfidence" effect.[2]
Modafinil is becoming increasingly popular among the general population.[8] Apart from a consumer's want to increase his neurological performance, there are financial incentives for manufacturers as well. Modafinil has a market share of more than $700 million a year, indicating a high degree of off-label use.[4] Modafinil is also one of the more easily available neuroenhancement drugs in the market today. Modafinil can be bought from many websites – mostly from Asian countries – as well as from darknet markets.[4][72][73] Modafinil first came into public attention when world champion runner Kelli White was tested positive for illegally consuming modafinil in the Athletics World Championship in 2003, resulting in the loss of her two gold medals.[4]
For research comparing modafinil with similar compounds, investigating combinations and showing trade-off type issues, see #Research topics below.
Methylphenidate
Methylphenidate (MPH), also known as Ritalin, is a stimulant that is used to treat attention-deficit hyperactivity disorder (ADHD). MPH is known to be highly abused by the general population, especially college students.[2][4] In an online poll conducted by Nature magazine, 12.4% of 1400 corresponding readers admitted use of MPH for non-medical reasons. Their reasoning behind its use was for increasing concentration, sleep deficit, and jetlag.[2]
A comparison between the sales of MPH to the number of patients revealed a disproportionate ratio, indicating high abuse.[2] MPH is believed to have a positive effect on memory consolidation, but studies have not been able to conclusively verify this claim.[2][10] Popular opinion that MPH enhances attention could not be verified.[2][10] Studies of MPH have reported improved problem-solving skills. However, when these studies were repeated to replicate the results, the placebo group scored higher, indicating that MPH may even impair performance.[4]
These inconclusive, and generally negative, results for memory improvement are insufficient to explain the use of MPH for non-medical reasons. Users may have motives other than genuine neuroenhancement that propels its unprescribed use, such as subjective and recreational effects.[2] The lack of any result, positive or negative, indicated that the 10–20 mg dosage may be too low for the drug.[2] Further studies need to be conducted, looking at different doses of MPH.[2][10]
Memantine
Memantine is an NMDA receptor antagonist and is used to treat patients with moderate to severe Alzheimer's disease, but is also used as a neuroenhancement drug.[3] Studies conducted on memantine were unable to conclusively verify neuroenhancement capability of the drug. Since most of these studies were single-dose tests of memantine, it is possible that these drugs would only show some effect, positive or negative, after continuous intake. Until then, single-dose studies of memantine are not enough to reveal the drug's actual potential.[3]
Donepezil
Donepezil is an acetylcholinesterase inhibitor (AChEI) that is used to treat patients with mild to moderate Alzheimer's disease. While many AChEIs could be potential neuroenhancement substances, donepezil is the most commonly used AChEIs by the general population due to its widespread use for treating Alzheimer's disease.[3]
Most studies on donepezil are unable to conclusively verify the neuroenhancement capability of the drug.[3] In such studies, it was seen participants who took donepezil scored higher than those that took the placebo. Donepezil helps individuals retain training tasks, verbal memory, and episodic memory.[3] In sleep deprivation studies, while donepezil had no effect on well-rested patients, it had a positive effect on patients with 24 hours of sleep deprivation. Such patients benefited from increased memory performance and attention that would otherwise be a deficit in such sleep-deprived conditions.[3] However, this effect was only seen in individuals whose performance declined significantly due to sleep deprivation.[3]
Research and candidates
Research also explores derivatives of already existing cognitive enhancers that have or could have higher bioavailability, such as N-acetylcysteine amide for NAC[75] and other bioavailability-enhancing strategies.[76] Another approach to enhance efficacy, potency or selectivity that is relevant to medications in general is improving drug delivery,[77][78] including enabling additional routes of administration such as via nanoemulsions for "nose-to-brain" drug delivery[79] or hypothetically via brain implants.[80]
Differential half-lives may also be a topic of research and development. Modafinil substantially increases alertness but, having a long half-life of approximately 13 hours,[81] can delay or impair sleep-onset,[82][21] with there being no marketed shorter-acting version. According to two 2009 studies, armodafinil is eliminated approximately three times more slowly than the S-isomer of racemic modafinil.[83][81]
Research may also investigate:
- general safety and efficacy in healthy people in particular[43][84][85][7][41] as well as for long-term use[58][85][35] and also using neuroimaging[85] (and associated brain mapping[86] and neuroimaging intelligence testing)
- Studies may also measure impacts of regular consumption of chemicals like modafinil on mortality and lifespan in animal models, such as short-lived mice.
- different protocols (e.g. dosages,[61][7] timing and scheduling)
- combinations[77] (e.g. concurrent, cyclic or sequential and precursors, depletions[87][88] and cofactors)
- One common combination under research is the concurrent combination of l-theanine with caffeine.[89][90][91]
- One study investigated high-dosage modafinil combined with low-dosage caffeine – 200 mg of each.[92]
- There are also studies combing different types of cognitive enhancers such as biochemical and behavioral enhancers such as modafinil combined with meditation.[93][94]
- The multinutrient intervention of uridine combined with choline (CDP-choline or e.g. in eggs) and omega 3 (e.g. in fish) has synergy, improving some measures of cognition and mood better.[95][96][97]
- factors of effects outcomes (including situational, task-difficulty,[98] personal (e.g. genetic[7] or baseline-skill-levels),[61][98][62][41] protocol-related, etc)[33][7]
- interactions (e.g. polypharmacy and combinations)[26][77]
- trade-off-type issues[33][99][98][41]
- Modafinil can, depending on various factors, at or around the time of consumption:
- impair sleep due to its long half-life[81][82][21]
- (slightly) increase the resting heart rate and blood pressure[12][100]
- impair some types of creativity[1][33] but possibly be beneficial[1] in some creativity domains or creative tasks (like e.g. literary fiction writing)
- both decrease or increase anxiety[101][102][103][1]
- have a possible effect of overconfidence[2][98]
- have risks for side effects like headache[1][100] and have low abuse/addiction potential[1][100]
- Some pharmacological agents may have issues due to which some may consider them as not viable for enhancement or not consider them to be neuroenhancers – for example "nicotine and amphetamines (such as Adderall)" may result "in substantial loss of cholinergic and dopaminergic receptor responsivity and may ultimately lead to their epigenetic downregulation" .[44] Various recreational drugs are partly used due to cognitive enhancement effects[26][37][104][58] on e.g. sociability, mood and/or creativity – in the exemplary case of alcohol this can have substantial effects on health, including long-term negative consequences on cognitive functions.[37] They can also induce tolerance for the effects and addiction could develop from long-term drug instrumentalization.[104]
- Some domains of cognition that may appear unequivocally desirable to modulate such as mood may be beneficial – albeit not preconditions – for other cognitive functions: for example, phases of depressed mood or depression was often associated with enhanced creativity.[105]
- Enhancing memory for example may come at the cost of other cognitive domains and if it is for example enhanced during the wrong times, resultant memories may be more "noisy".[86] Forgetting and/or low memorization may be beneficial with current constraints and enhancing either could be beneficial in many situations or nonpathological issues.[41][60] There also is the concept of "enhancement through diminishment" that via addition-by-subtraction interferes with the function of brain regions that are. less essential or counter-productive to task performance. This concept is not to be confused with the "cognitive diminishment" described in #Cognitive diminishment and the "neurodiminishing" described below.[60]
- There is a possibility that in the future the use of neuroenhancement tools is exploited by adverserial forces or malicious entities to negatively influence performance, called "neurodiminishing"[60] – potentially a form of neurological warfare (also called "neurowarfare"; overlapping or synonymous with "cognitive warfare")[106][107][108][109][110][111] beyond the enhancement of troops' capacities,[112][113][114] for example via electronic warfare,[60] hacking,[115] directed energy sources,[116][60][106][117] manipulation,[118] or other methods.
- Modafinil can, depending on various factors, at or around the time of consumption:
- differential effects (e.g. comparisons between substances/strategies[33] and per task, cognitive domain[33] or purpose)
- For example, it was found that methylphenidate (Ritalin) increases alertness more on simple tasks than on difficult ones, while modafinil increases concentration or attention,[21][2] reportedly may significantly outperform methylphenidate "for cognitive enhancement in healthy individuals, 'especially on people undergoing sleep deprivation'",[119] and is thought to also have an impact on decision-making, planning, moral reasoning[120][121] and motivation.[42][38][39][122] Modafinil has replaced dextroamphetamine in certain types of military operations due to its superior side effects profile.[92]
- development of more adequate tests, evaluations or measures about positive effects
- A study notes that there is a problem of "how to define cognition and whether or not it can be understood in simple computational terms", calling for "studies testing whether putative enhancers improve the performance of sophisticated (highly experienced) subjects dealing with novel circumstances of great complexity and without the benefit of external supervision", which could "bring experiments closer to the human condition and thereby help explain why animal studies on cognition and memory have such a poor record in predicting human outcomes".[86]
- Neuroimaging could also be used, including for analysis of long-term impacts.[85]
- use of and/or more advanced and/or more accessible technologies for "nutrition assessment (eg, metabolomics and innovative methods of dietary intake assessment) and recently identified biomarkers of nutrition and neurobiological outcomes" for prevention of cognitive impairment as in screening[123]
- general underlying neurobiology such as mechanics and variations underlying types of creativity or attention and focus (attentional processing) – e.g. neurotransmitter systems, neuropeptides, gene expression, neurotrophins, brain metabolites, neuroimaging/network-mapping/neuroanatomy, etc[44][105][124][125][86]
Intrabrain bioengineering
Advanced cognitive enhancement that is not viable for use by humans in the near future could build upon research in which receptors to activate or inhibit neurons with proteins were designed, e.g. using "Designer Receptors Exclusively Activated by Designer Drugs" (DREADD).[126] Genetically modified neurons may enable connecting external components to nerves.[127] Researcher reported in 2020 that they bioengineered C. elegans worms to synthesize, fabricate, and assemble bioelectronic materials in its brain cells. They enabled modulation of membrane properties in specific neuron populations and manipulation of behavior in the living animals.[128][129][130]
If organic neuromorphic devices reach a certain point and become biocompatible, novel brain implants could be possible.[131] There also is research of potentially implantable[132] physical artificial neurons.[133] Bioimplants of genetically engineered or stem-cell grown neural tissue may become possible as well.[134] Another approach are wetware computers.
Dietary components and supplements
Various compounds contained in foods (sometimes called brain foods) or consumed in isolated forms or herbs such as cinnamon,[135] cocoa powder,[136][45][137][138][91] anthocyanins (e.g. in bilberry and black elderberry),[139] dietary nitrate (in beet root),[91] honeys,[140] polyphenols (in many fruits and vegetables),[91][141][142][143] epicatechin,[144][145] levodopa[43] l-phenylalanine[146][147] and l-tyrosine,[148][87] phenethylamine (PEA),[149] carotenoids like lycopene (in tomato sauce),[149] l-theanine,[150][89][90] apigenin (and chamomile),[151][152] ginger,[153][138][147] herbal infusions (notably lemon balm, rosemary, peppermint and caffeinated drinks),[154][155][156][157][26][147] rhodiola rosea,[77][85][147][158][159] creatine,[160][161][162] omega 3 (e.g. sustainably algae-derived),[167] as well as correction of prevalent micronutrient deficiencies[168][169][170][171][147][172] are investigated for potential minor but significant or additive impacts on cognition (e.g. mild stimulation and/or mood modulation) in healthy non-old individuals.
The dietary component glucose (and its glycogen form) is the main energy source for the brain, with some researchers considering it a "[b]iochemical enhancer" despite of the health impacts of direct consumption. A constant supply of it is needed and simple sugars can spike blood glucose and their glucose supply does not last long. Slowly absorbed carbohydrate-containing food or low-GI food would lead to a slower release of glucose than a quickly absorbed or high-GI food.[173][91][33][147] There is very little research on links between brain glucose metabolism and cognition, despite it also being relevant to neurodegenerative disorders.[174] Lactate (released especially during specific types of exercise) may also be relevant to cognitive enhancement.[175][176][177]
Medications
Notable potentially viable pharmacological agents – as final products or as prototypes for similar ones – under early-stage research with potential for substantial effect sizes for specific purposes in specific situations (such as learning periods) also in healthy non-old humans but, in at least most cases, largely unknown effects in humans and safety profiles (and consequently not widely used or not used at all): orexin-A,[119] FGL, PTEN-PDZ, and PI3K-activator PTD4-PI3KAc,[178] dihexa,[179][180][181] d-cycloserine,[182][183][184][41][185] DAT blockers CE-123 and CE-158,[186] ampakines like IDRA-21 and CX717,[187][188][41][147][189] rapastinel,[190][191][192] ISRIB,[193][194] citicoline,[195] selective receptor modulators such as MRK-016 which targets subtypes of GABAA receptors,[196] modafinil-analogs CRL-40,940 and modafiendz,[100] modafinil-inspired/hybrid TRIs JZ-IV-10 and JZAD-IV-22,[197][198][199] TAK-925 (orexin agonist that promotes wakefulness in ways similar to modafinil),[200][201] wakefulness-inducing or procognitive narcolepsy medication candidates like samelisant,[202][203] pterostilbene,[204][205] CRM acyl-ghrelin mimetics and agonists like ibutamoren,[206][207] and H3 receptor antagonist pitolisant.[208][202][209]
Some medications like widely used sociability-related GABA receptor agonist phenibut can have durable major side-effects and addiction potential for some at least at some dosages, albeit not necessarily.[210][211][48][212] Other notable repurposed chemicals already widely in use for other purposes and with potential viability for neuroenhancement but in most cases no or few/small trials with healthy non-old humans, limited research on side-effects and/or unvalidated effect-sizes include: CBD,[213][214] neuropeptide cerebrolysin,[22][215] nicergoline,[216][85] huperzine-A,[217] DMAE and meclofenoxate,[85][217][35] vinpocetine,[85][217] palmitoylethanolamide (PEA),[218] and pyritinol.[219][85]
Non-pharmacological
Neurostimulation
Neurostimulation methods are being researched and developed. Results indicate details of the stimulation procedures are crucial, with some applications impairing rather than enhancing cognition and questions being raised about whether this approach can deliver any meaningful results for cognitive domains. Stimulation methods include electrical stimulation, magnetic stimulation, optical stimulation with lasers, several forms of acoustic stimulation, and physical methods like forms of neurofeedback.[33][126] There are ideas to integrate such headgear into helmets.[119][126]
Transcranial direct current stimulation
While neuroenhancement drugs are a potential method for cognitive performance enhancement, transcranial direct current stimulation (tDCS) over the motor cortex (MC) is being seen as another potential method.[220] Although it was originally intended to help patients with brain injuries such as stroke, there has been a lot of interest in the last few years on tDCS's capabilities for healthy individuals as well. Recent studies have already shown improved neuroplasticity from tDCS to facilitate motor learning in young humans, and it may be possible to apply this method to the older segment of the workforce as well.[220]
Stimulating higher cognitive functions of the brain, such as the language function, with tDCS in one study resulted in improved word retrieval. tDCS works by enhancing the connectivity in a given stimulated network, providing neural efficiency in highly specific brain areas critical for task performance.[221] During this time, fMRI images also showed reduced activity in the semantic retrieval processes, suggesting more efficient processing in task-critical areas of the brain.[221] Reduced activity in circumscribed task-related areas has been attributed to consolidation of motor learning and superior memory performance. New research in tDCS is trying to localize the stimulation to affect the desired subset of highly specific task-relevant neurons.[221] In 2022, scientists demonstrated that transcranial alternating current stimulation (tACS) can, depending on the frequency, for one month improve (either) short-term memory or long-term memory in 65–88-years-old people.[222]
Deep brain stimulation

Deep brain stimulation (DBS) is another form of neuroenhancement. Unlike tDCS, though, DBS involves the implantation of a medical device, and is restricted for use for only a few, severe diseases such as Parkinson's disease and dystonia.[223] In one study, DBS improved movement by 39%, reduced disability by 38%, and improved quality of life by 30% for patients with dystonia over a course of 3 months.[223] The patients had a reduction in dystonia symptoms by 50%.[223] Improvement was noticeable within hours to days after DBS application. The benefits of DBS as of now are far more than those of high-dosage trihexyphenidyl, a powerful drug used in the treatment for dystonia.
Brainwave entrainment
Brainwave entrainment, also referred to as brainwave synchronization or neural entrainment, refers to the observation that brainwaves (large-scale electrical oscillations in the brain) will naturally synchronize to the rhythm of periodic external stimuli, such as flickering lights,[224] speech,[225] music,[226] or tactile stimuli.
As different conscious states can be associated with different dominant brainwave frequencies,[227] it is hypothesized that brainwave entrainment might induce a desired state. Researchers have found, for instance, that acoustic entrainment of delta waves in slow wave sleep had the functional effect of improving memory in healthy subjects.[228]A study showed that a "visual flicker paradigm to entrain individuals at their own brain rhythm (i.e. peak alpha frequency)" resulted in substantially faster perceptual visual learning, maintained the day following training. In particular, the entrainment substantially accelerated learning (this group "improved at least three times faster than control groups") in a discrimination task to detect targets embedded in background clutter or to identify radial vs. concentric Glass patterns embedded in noise compared to entrainment that does not match an individual's alpha frequency.[229]
Conventional methods and foundational strategies
Enhancement can also be based on conventional methods; this may be considered neuroenhancement[230] if they are targeted, especially when they are applied to an advanced level or if they are considered from a collective public health intervention[230] perspective. From this perspective, "failing to encourage the pursuit of healthy behaviours" has "adverse effects on population cognitive health".[230]
Conventional methods include those that delay or mitigate brain aging (which is one major preoccupation of neuro-enhancement),[231] including adequate sleep, optimized diet, and physical activity (which has many neuroeffects).[230][232][56][24][22][38][23]
However, they may also be considered as "co-strategies" in parallel to "neuroenhancement", rather than as techniques of cognitive enhancement.[233] Conventional cognitive enhancement methods and "more direct neuromodulatory methods" could be used together[57][134] (e.g. environmental enrichment and nootropics)[125] for desired effects.
Cognitively stimulating and social activities can also have positive effects on the brain.[23]
Environmental factors
Environmental protection measures can also protect or enhance cognitive abilities – for example studies have well-validated extensive harmful effects of ubiquitous air pollution (outdoor[234][235][236][237] and e.g. PM2.5 and CO2 concentrations indoor[238]) and shown that half of the US population has been exposed to substantially detrimental lead levels in early childhood (mainly from car exhaust whose lead pollution peaked in the 1970s and caused widespread loss in cognitive ability).[239]
Education- and time-use-related
Development of education may also be part of neuroenhancement. Results in neuroscience and software – e.g. AI and adaptive online learning environments – can be relevant to this development.[240][241][242][243] Lifelong learning may be considered a way of cognitive enhancement.[42] For the scope of neuroenhancement relating to education and work, see #Scope for cognitive enhancement.

How people spend their time or activities may have major effects on cognition[23][245][28] (and vice versa) and be modulatable in various non-pharmacological ways such as decision-making, prioritization, routines, reflective practices, reasoning-related technologies, gamification,[42] incentives (e.g. economics, economic policy, media policy, curricula, social feedback, norms, etc), and so on. One review suggests that including motivation enhancement measures "such as motivational interviewing and the use of rewards or incentives" makes interventions substantially more successful in achieving improvements in health behaviours.[246] Exemplary activities include sleep, wage labour work, school, social media, TV, volunteering, boredom, exercise, social activities and hobbies.
Time-use
There is time-use research and research on various types of media uses on cognition. Impacts of screen time as well as play behavior on cognition may depend heavily on the activities, contexts, substituted activities and contents.[245][247]
Motivations to make use of pharmacological cognitive enhancers include "time optimization" and "increase in time awake".[21] Time requirements for neuroenhancers may be an important factor in their selection or adoption.
Education and cognition
Integration of digital tool use may increase cognitive capacity and flexibility, lower cognitive load and foster digital fluency.[248][243] Skills in critical thinking, technology-supported inquiry learning,[249][250][243] scientific reasoning abilities (e.g. compare with religious education and the "cognitive style of religious thinking"[251][252]) and problem-solving may be related to the cognitive domain or represent "metacognitive skills".[253][254][243][255] Improvements to education could be considered cognitive enhancements and "educators" may commonly commit to a fallacy whereby it is assumed that if "individual distinct cognitive processes can be enhanced [...] [this] must enhance cognition overall" when they deploy "'teaching to the test'" and prioritize "memorization over generalizable skills such as critical thinking and problem solving".[256]
Another facet of education from a neurological perspective is integration of neuroenhancement options (behavioral, pharmacological, or devices) in learning processes in specific or in various types of training or education (e.g. by students, retrainees or military personnel). This could be considered as part of evidence-based learning and is concerned with ensuring applied methods are safe, effective and are used in the right way – potentially including personalization – for the right purpose(s) at the right time(s) during the learning process(es). In contrast, a DIY-approach , rather than guided ones, requires at least extensive research by the person seeking to enhance aspects of cognition.
Software and media
Educational software may also fall into the scope of neuroenhancement (which may depend on the kinds or types of use or features of such).[22][42] Applications of augmented reality technologies are investigated for general memory enhancement, extending perception and learning-assistance.[257][258][126]
The Internet is sometimes considered as a "powerful cognitive enhancement technology"[241] or as enabling "Internet-extended cognition" or "Web-extended minds" or "human-extended machine cognition".[259][260][261] However, it is not "a simple, uniform technology, [n]either in its composition, [n]or in its use" and as "an informational resource currently fails to enhance cognition", partly due to issues that include information overload, misinformation and persuasive design. Substantial neuroenhancement potential therefore may lie in measures such as individual empowerment (possibly via existing education systems), software development and better collaborative systems for sorting and categorizing information.[241]
One trial investigated a smartphone motivation enhancement application "promoting lifestyle improvement for brain health".[262]
What could be described as "human-computer symbiosis" already permeates daily life, including for example humans' use of Web search software for research or machine translation.[263][264] A "gradual transition from document-centric to more data-centric modes of information representation", as envisioned by Semantic Web developments, could "provide new opportunities for cognitive augmentation and enhancement". This would enable targeted retrieval of specific pieces of task-relevant information and highly flexible modes of information display, in a way that is more advanced or integrated than conventional desktop computer Web search querying,[265] which is further complicated by that enhancement of the reasoning- and inquiry-skills[243] of Web research, media literacy and sophisticated use of literature- and Web-search engines and tools are typically not part of curricula.
Downsides of "merger[s]" with Information and communications technologies (ICTs), often called or misidentified as "AI" in the literature and media, may currently include "loss of privacy, political polarization, psychological manipulation, addictive use, social anxiety and distraction, misinformation, and mass narcissism"[266] and cause – societally detrimental forms of – harm to mental health and well-being.[267] ICTs and AI tools have enhanced many human skills and practices in ways not feasible without such. For example, AI can enhance artists' creative process or creativity.[267] It has been speculated that AI could monitor and advise via factors that are relevant to or affect moral decision-making as one form of moral enhancement.[268] Externalizing or "offloading" information into an external artefact, especially digital ones, allows people to materialize their thoughts, thereby overcoming the limitations of brains.[269] Whether or not technologies are currently "enhancing the way we think and reason, and thus [are] making us smarter" or not (cognitive diminishment) is a topic of debate and research (or more precisely determinants, factors and impacts),[270][269] and may depend on changeable factors such as:
- whether their design is public[270]
- ethical frameworks for design[270]
- policy
- systematic incentives and mechanisms of the contemporary socio-economy which facilitate or determine their use/adoption, patterns of use, success/production, creation/feasibility, and quality
- incentives and mechanisms that are built into the technologies or inherent to them (these may be interdependent with surrounding infrastructures, transparency/product information, standards, design- and policy-decisions that are based on the prior socioeconomic incentives, assessments-/ratings-mechanisms, life-cycle analyses, and metadata)
Early childhood
Nutrition also plays an important role during early brain development.[166][169][271]
A 2021 study showed that the Abecedarian Early Intervention Project resulted in significant changes in midlife brain structure in males. MRI scans showed that several brain regions' and total brain volumes were substantially larger in participants of the childcare program than in the control group.[272][273]
Protection from pollutions (such as direct and indirect vehicular pollution) appears to be of special importance during and before early childhood,[278] with age being the largest factors that affect neurotoxic outcomes for a given pollution.[235]
Concerning aforementioned screen-use, children's exposure to computers as part of school curricula is important to the development of computer literacy.[279]
Play behavior may also extend to the choice of toys, as social-emotional and cognitive skills are developed and enhanced as children play. A study recommends toys that encourage the child to be mentally and physically active.[280] For example, several construction toys like Meccano could facilitate children to learn skills that are embedded in the act of designing and creative thinking.[281][282] Stimulation of creativity during childhood "enhances cognition and even can alter the brain both anatomically and physiologically".[105]
Other
Correction of mild dehydration[91] (which may be especially relevant when chemicals like modafinil have been consumed), listening to various types of music and other audio[283][284][12] (e.g. ambient music, binaural beats or soundscapes for focus and other types for mood, motivation or alertness) or noise-reduction,[12] and intake of – at least specific – fruits (and possibly nuts)[285][286][287][91][288][77] can have immediate (acute) significant effects depending on various factors.
Targeted microbiome alterations such as via various psychobiotics,[294] meditation,[33][28][295] and physical relaxation techniques like cyclic breathing exercises,[296] could have longer-term effects, albeit the research on these interventions is at an early stage with few human trials[291] – which may be needed to, for example, identify beneficial bacterial species and strains to include in a probiotic – despite a study reportedly first showing that the gut microbiota can be a therapeutic target for cognitive enhancement in the year 2011.[297]
Research also investigates differences between adults' learning and children's learning which may enable interventions to selectively enhance learning in adults and children.
Sleep-related
The role of sleep in learning can be leveraged by interventions such as "targeting specific neurotransmitter systems pharmacologically", "stimulating sleep-specific brain oscillations" and "cueing memory reactivation during sleep", including via "[o]lfactory and auditory cues".[184]
Desynchronized circadian rhythms have detrimental effects on cognition.[298] In learning and skill development, the sleep episode following initial skill practice is important for consolidation.[299] Sleep evasion which may be useful for increasing time available and wakefulness can be studied in a variety of ways.[189] Sleep is investigated for potential ways to leverage it for acutely (e.g. via complex analogical problem solving or sleep onset periods as creative sweet spots)[300][301] or chonically[301][302] improving types of creativity.
There is research into interventions to enhance sleep as in improving sleep quality, efficiency (sleep latency), and duration (e.g. 7–8 hours for most adults in specific but depending on factors that include genetics, possibly (preceding) exhausting physical activity[303] and possibly factors of "perceived sleep needs"[303]).
Candidate tools or targets for modulation (technological,[304] legal, pharmacological, or otherwise) include glycine, melatonin (which can have side effects), diet and exercise, post-waking cognitive effort such as via alarm clocks that are deactivated by solving simple puzzles,[305][306][307] neuromodulation sleeping caps,[308] lemon balm,[309] tryptophan, valerian, ashwagandha,[310][311] caffeine-[312][303] and meal-timing, circadian rhythm adherence, lightning, screen-use and blue light reduction before sleep, and various optimal sleep environment characteristics[313][314] (which may include indoor air quality, thermal properties of bedding,[299] temperature,[299][303] noise,[303] and outdoor light at night).
Augmented reality
Applications of augmented reality technologies are investigated for:[257][258][126]
- general memory enhancement
- providing "just in time" information[315]
- parallel quadcopter operation
- enhancement to detect, classify, and successfully manage (e.g. engage) threats[316]
- spatial awareness[316]
- enhancing visual and multisensory perception[316]
- learning-assistance
Challenges for adoption include "short- and long-term risks of distraction, mental workload, visual occlusion, and technology-induced complacency and skill degradation".[316]
Genetic enhancement
A potential future biochemical strategy for cognitive enhancement is human genetic enhancement which has only been preliminarily, but successfully, tested in animal models and is currently not an available enhancement option to researchers.[33][28][317]
Candidate target genes
A neurogenetics genome-wide association study (GWAS) meta-analysis investigated genetic correlations of language-related skills, reporting genetic factors of, the so far uniquely human, language-related capabilities, in particular factors of differences in skill-levels of five tested traits. It also used neuroanatomy/neuroimaging data.[320] However, correlations are not to be equated with causal factors and causal factors are neither independent from surrounding factors of society nor necessarily exclusively beneficial.
Side effects
Common neuroenhancement drugs are typically well tolerated by healthy humans.[2][3] These drugs are already in mainstream use to treat patients with different kinds of psychiatric disorders. Since most of the information on neuroenhancements and their capabilities are drawn from research experiments, the best way to determine adverse effects are drop-out rates and subjective rating.[2][3] The drop-out rates were minimal or non-existent for donepezil, memantine, MPH, and modafinil.[2][3] In the drug trials, participants reported the following adverse reactions to the consumption of donepezil, memantine, MPH, or modafinil: gastrointestinal complaints (nausea), headache, dizziness, nightmares, anxiety, drowsiness, nervousness, restlessness, sleep disturbances, and insomnia.[3] The side effects normally ceased in the course of treatment.[3] Although there were no reported side effects from DBS, 18% of the patients reported device-related complications such as infections due to lead dislodgment or breakage.[223] Various factors such as dosage, timing and concurrent behavior may shape or determine the appearance of side-effects. There may be risks of dehydration (inadequate concurrent hydration).[12] However, there also is widespread usage of cognitive enhancers whose long-term and/or short-term effects on health are nearly entirely unknown or which are known to be unhealthy.
Ethical, social and legal issues
Adverse health impacts and dependence
Parents and healthcare providers are concerned about the safety and well-being of those that consume various neuroenhancements.[322]
In a 2011 article by Jayne Lucke, the concept of neuroenhancement is compared to sildenafil. The author states that "recreational users of [sildenafil] had lower confidence in their ability to achieve an erection than non-users, even though they had a significantly better erectile function. They become psychologically dependent on these drugs." The author believes a similar issue can be seen in neuroenhancement users.[322] Moreover, expectations or overestimations regarding the effectiveness of interventions can exceed their actual effects and they can induce overconfidence.[2][24]
If more and more people begin enhancing their minds, people may "eventually feel subtly coerced into enhancing themselves in order to remain competitive in school or the workplace" or in the military, or experience types of peer-pressure.[119][323][47]
Dubljević stated that "making sure that users are well informed, tracking any adverse effects and generating funds (e.g., via taxation or fees) to address related social problems is crucial", suggesting governments could also play a more active role in the addressing of neuroenhancement challenges.[324]
In health systems where production does not sufficiently adjust as in increasing production and reducing production-costs, neuroenhancement may lead to "prescription drug diversion".[35] One review hypothesizes that the limited number of recent randomized controlled trials with healthy people in particular could partly be explained by (the research being influenced by) the media and bioethics literature surrounding pharmaceutical cognitive enhancement in healthy subjects.[7]
Validation and quality control


There appears to be a lack of various requirements, quality standards, validation and authentication, sampling and lab testing for cognitive enhancers including dietary supplements, (other) chemicals and (other) therapeutic goods,[325][326][327][328][329][330] which may be the result of public safety-governance failure.
This means that if a user is perfectly aware of the current state of science on the risks and benefits of a chemical and personal factors, there can still be risks that the product consumed differs from its label[331] or has a lower quality which in some cases can be dangerous,[332] especially if such are not available through large established shops which have more to lose,[333] assuming such threats to safety are always unintentional and limited to producers and/or retailers (like online pharmacies).
One study raises the question of creating "a knowledge base as part of nutrition and health education in schools and adult education to enable people to counteract marketing and advertising related food supplements, as well as to distinguish meaningful from non-efficacious ingredients or at least to scrutinize products for their potential health risks".[334]
Well-being and productivity
The ethical benefits of neuroenhancement include potentials to:
- improve well-being[43][335][134]
- reduce unhealthy substance use[52][53][55]
- further educational aims[134]
- increase societal productivity[99][336][337][134] in constructive sectors (which is also relevant to aforementioned well-being)[336][134]
- support socioeconomic shifts by "enabling more complex, productive, and inherently fulfilling forms of cooperation"[134] (which may also be relevant to aforementioned well-being)
- enable self-amelioration of widespread mild cognitive deficiencies in cognitively normal individuals[336]
- increase incentives for and the effort behind the development of medical therapies that can benefit people affected by various brain diseases, and in some cases other diseases, such as Alzheimer's disease, and by brain aging. Various cognitive enhancement options – e.g. dietary and/or pharmacological ones – could also be protective against such pathologies (as in preventive healthcare).[338]
Diversity and inequality
Neuroenhancement is often seen analogous to the issue of doping in sports, due to which it is sometimes called brain doping.[322][23][210][25][58] A common concern raised is an unfair advantage of people who consume enhancing drugs over people who don't. Many athletes, however, feel that the only way for them to win against athletes that take performance-enhancing drugs (PED) is for them to take PEDs as well; a similar thought process has developed within the general population in regard to people that consume neuroenhancement drugs.[322] In a research study of 18- to 34-year-olds, 50% of them had little or no objection to the concept of doping.[322] Students, in particular, often feel that cognitive neuroenhancers are acceptable.[322]
Generally, the moral acceptability (including fairness perceptions) of such substances for the purpose of neuroenhancement are an important factor in the decision to use or not use such drugs. Studies found that moral objections against such substances strongly decrease the willingness to use them.[339][340][341]
Many argue that the only option for regulation of neuroenhancements is to allow it to everyone, thus minimizing cheating. Banning the drugs, on the other hand, may have detrimental consequences to society.[8] Not only would it create a black market, amplifying issues caused by illicit use, it would also increase the cost to society from enforcing the law.[322] Neuroenhancement drugs need to be assessed further for their merits and adverse effects, making it easier for policy-makers to make a call on the regulation of such drugs.[10]
In general, cognitive diversity – or some "optimum range of diversity" – was found highly valuable. Novel capabilities due to progress in science and technology may raise related ethical issues.[342] There have also been speculations that cognitive enhancement technologies (CETs) may increase population-level cognitive diversity, e.g. as different people will choose to enhance different aspects of their cognition.[343] Moreover, cognitive enhancements might decrease inequality, e.g. by "leveling the playing field"[344] as well as narrowing the inequality of the "genetic lottery".[7]
Distributive justice
Another issue is that of distributive justice, concerned with "who will have access to new cognitive enhancement techniques, and who can experience the cognitive benefits".[323][99][134][35]
A main factor in the costs of cognitive enhancers is their patentability.[33] Online pharmacies and online shops can substantially reduce costs, raise affordability or enable consumers to purchase certain products at all.[329][345][346] Naturally occurring agents (contained in small amounts in foods) may offer "more options to patients who may be of poor socioeconomic backgrounds, or are residents of poorer nations".[77]
Allen Buchanan in a book suggested that "we should embrace the opportunities provided by this emerging pharmacological technology and devote our resources to ensuring that such drugs that are developed and tested properly [e.g. see confounding, reproducibility, #Research topics and cognitive test] and that access to them is equal and open to avoid injustices and the development of black markets".[347]
Cognitive liberty and autonomy
Where the first obligation seeks to protect individuals from interference with cognitive processes by the state, corporations or other individuals, this second obligation seeks to ensure that individuals have the freedom to alter or enhance their own consciousness.[348] An individual who enjoys this aspect of cognitive liberty has the freedom to alter their mental processes in any way they wish to, whether through indirect methods such as meditation, yoga or prayer, or through direct cognitive intervention through psychoactive drugs or neurotechnology.
As psychotropic drugs are a powerful method of altering cognitive function, many advocates of cognitive liberty are also advocates of drug law reform, claiming that the "war on drugs" is in fact a "war on mental states".[349] The CCLE, as well as other cognitive liberty advocacy groups such as Cognitive Liberty UK, have lobbied for the re-examination and reform of prohibited drug law; one of the CCLE's key guiding principles is that "governments should not criminally prohibit cognitive enhancement or the experience of any mental state".[350] Calls for reform of restrictions on the use of prescription cognitive-enhancement drugs (also called smart drugs or nootropics) such as Prozac, Ritalin and Adderall have also been made on the grounds of cognitive liberty.[351]
This element of cognitive liberty is also of great importance to proponents of the transhumanist movement, a key tenet of which is the enhancement of human mental function. Wrye Sententia has emphasized the importance of cognitive liberty in ensuring the freedom to pursue human mental enhancement, as well as the freedom to choose against enhancement.[352] Sententia argues that the recognition of a "right to (and not to) direct, modify, or enhance one's thought processes" is vital to the free application of emerging neurotechnology to enhance human cognition and that something beyond the current conception of freedom of thought is needed.[353] Sententia claims that "cognitive liberty's strength is that it protects those who do want to alter their brains, but also those who do not".[352]Human rights
A new human right to cognitive liberty has been proposed by researchers earlier[354][355] and in a 2023 book by Nita A. Farahany as "an update to other existing human rights to privacy, freedom of thought and self-determination", partly because some neuroenhancement technologies could possibly also be used for ways like "involuntary neural surveillance" (including for business purposes), be vulnerable to hacking or used for manipulation.[356][357][358] Others have suggested "the right to cognitive liberty, the right to mental privacy, the right to mental integrity, and the right to psychological continuity".[354] In 2021 Chile became the first country to approve neurolaw that establishes rights to personal identity, free will and mental privacy.[359]
Scope for cognitive enhancement
Major preoccupations of neuro-enhancement include optimization of child development factors and delaying, reversing or mitigating brain aging.[231] Attempts to enhance human memory and learning ability have a long history in science (and proto-science).[361]
Elderly

Proponents of cognitive enhancement have argued that there are vast potential benefits for the workforce, especially for the older segment.[220][77] Mainly due to advances in medical technology over the last century, the average human life expectancy has increased significantly. Demographics for developed countries indicate rapid growth of the older segment of the workforce. Advancing age generally shows a pattern in the reduction of the ability to acquire new skills, but integration in the industry today requires employees to be able to acquire and retain new skills more than ever before.[220] With an increase in the ageing population comes an increase in the economic, social, and health burden of age-related disorders and diseases.[77] Enhancing cognitive functions has recently gained substantial increases in attention "because of the increasing percentage of older individuals worldwide and the predicted rise of age-associated cognitive decline in brain function".[361]
Healthy non-elderly
A review noted that "even healthy [non-old] individuals who normally function well, are not always performing normally due to sleep deprivation, jet lag, or other stressors, and some might need cognitive enhancers to perform at their best possible level on some occasions".[99] Neuroenhancement thus is concerned with improving capacities in various cognitive domains to approximate their best possible level at more and specific times as well as raising that level, and improving neuropsychological weaknesses or mild deficits that are not diseases.
Purposes of enhancement and behavioral interventions
Concerning public policy and neuroenhancement-related institutionalized structures like education systems, it is not known whether the scope of neuroenhancement also includes the purposes and content that cognition is enhanced for – the ends of cognitive enhancement such as curricula learned in educational institutions or ultimate purposes of tasks at workplaces or, by extension, the methods and frameworks by which such are e.g. selected. It also has not been clarified whether cognitive enhancement also encompasses behavioral interventions or methods (such as mnemonic techniques[33][28] like the method of loci[257]) and differential teaching modes and methods, albeit some studies indicate behavioral interventions fall into the scope of neuroenhancement.
Neurohacking and DIY
Neurohacking is a subclass of biohacking, focused specifically on the brain. Neurohackers seek to better themselves or others by “hacking the brain” to improve reflexes, learn faster, or treat psychological disorders.[363] The modern neurohacking movement has been around since the 1980s. However, herbal supplements have been used to increase brain function for hundreds of years. After a brief period marked by a lack of research in the area, neurohacking started regaining interest in the early 2000s.[364][365] Currently, most neurohacking is performed via do-it-yourself (DIY) methods by in-home users.[363]
Simple uses of neurohacking include the use of chemical supplements to increase brain function.[366] More complex medical devices can be implanted to treat psychological disorders and illnesses.[367]In neuroenhancement, the human brain is conceived of as a malleable "wetware".
Online communities
The Reddit forum (subreddit) on nootropics has many readers[368][369][370] and, along with many other related subreddits, enables communication including about topics or questions not yet studied directly or not easily findable in health information on the Internet, experience-based exchange, nootropic-based discussion, and other content for users of cognitive enhancers, which may include marketing. It is an unstructured accessible source of information from past and present users.[369] Researchers have analyzed reports on these websites, such as conducting content analysis of the subreddit r/microdosing.[40] One study used such an approach to highlight some of the gaps in the scientific literature, suggesting "main uses [of memantine] identified on reddit.com were not reported in the medical literature" and provided some data on the usage patterns reported there .[371]
Opinion
General public
The opinion of the general public on the issue of neuroenhancement is scattered.[322][372] In general, the younger population under the age of 25 feel that neuroenhancements are acceptable or that the decision lies in the hand of that individual. Healthcare officials and parents feel concerned due to safety factors, lack of complete information on these drugs, and possible irreversible adverse effects.[322] Such concerns have been shown to reduce the willingness to take such drugs.[373][374][340]
A 2016 German study among 6.454 employees found a rather low life-time prevalence of cognitive enhancement drug use (namely 2.96%), while the willingness to take such drugs was found in every tenth respondent (10.45%).[375] Studies have estimated that between 7–9% of the college population in the United States consumes neuroenhancement drugs. Some studies estimate this figure to be as high as 12% or even 20%.[372] A large-scale survey using a random sample of more than 5.000 German university students found a relatively low 30-days prevalence of 1.2%, 2.3% indicated the use of such drugs within the last 6 months, 3.2% within the last 12 months and during 4.6% during their lifetime, respectively.[373] Of those students, who used such substances during the last 6 months, 39.4% reported their use once in this period, 24.2% twice, 12.1% three times and 24.2% more than three times. It has been shown that consumers of neuroenhancement drugs are much more willing to also use them in the future, e.g. due to positive experiences or a tendency towards addiction.[341][376] Students primarily attribute consumption of these drugs for increased concentration, improved alertness, or to "get high".[322][372] Neuroenhancement drug users rated the positive potential of neuroenhancement drugs higher than non-users, and rated the adverse effects of these drugs lower than non-users, showing more confidence in the result of these drugs. In a survey of 1324 German students, 32% of participants that do not consume neuroenhancement drugs felt they had positive cognitive effects while 12% felt they had a relaxation effect.[372] In contrast, 54% of participants that do consume neuroenhancement drugs felt they had a positive cognitive effect while 25% felt they had a relaxation effect.
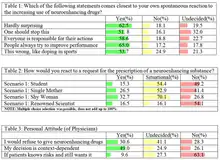
The need to remain "alert" and "focused" can also be seen in the trend of caffeine consumption. The caffeine consumption for both students and the general population of the US is around 90%.[372][377] Students who consume neuroenhancements also had a higher frequency of consuming psychoactive lifestyle drugs such as cannabis.[372]
A study among German university teachers (including professors) found a very low prevalence of neuroenhancement drug use.[341] Only 0.9% of the respondents reported the use of such drugs. However, 10% of the respondents are willing to take such drugs in the future, which might indicate a potential increase of the prevalence. One reason to use such drugs was work-related stress.
Physicians
Physicians play an important role in determining the potential abuse of neuroenhancing drugs. While some neuroenhancing drugs do not require a prescription and are easily available, others that require a prescription are up to the discretion of the physician. In a survey conducted among Swiss psychiatrists and general practitioners, the majority of surveyed physicians agreed that their criteria to determine whether or not a dysfunction should be considered a disease is if the patient indicates subjective suffering and/or negative consequences for the everyday ability to work.[6] The surveyed physicians, however, were in majority agreement that they do not prescribe medication without a clear indication of such a dysfunction.[6]
See also
- Bioeconomy#Medicine, nutritional science and the health economy
- Cognitive development
- Cyborg
- Development of the nervous system in humans § Adult neural development
- Intelligence amplification
- List of drugs used by militaries
- Neurogenesis
- Neuroplasticity § Types
- Neuroprotection
- Superintelligence § Feasibility of biological superintelligence
- Wearable technology
Drugs by psychological effects (15 C) |
Neuroprosthetics (1 C, 32 P) |
- Concepts and topics related to neurodiminishment
- Criticism of democracy § Criticism of process
- Dumbing down
- Dysgenics
- Indoctrination
- Internet manipulation
- Media studies
- Misinformation
- Neuromarketing
- Decline of civilization § Theories
- Other (currently not included) and sub-types
References
- Battleday, Ruairidh; Brem, Anna-Katharine (28 July 2015). "Modafinil for cognitive neuroenhancement in healthy non-sleep-deprived subjects: a systematic review". European Neuropsychopharmacology. 25 (11): 1865–1881. doi:10.1016/j.euroneuro.2015.07.028. PMID 26381811. S2CID 23319688.
- Repantis, Dimitris; Schlattmann, Peter (2010). "Modafinil and methylphenidate for neuroenhancement in healthy individuals: A systematic review". Pharmacological Research. 62 (3): 187–206. doi:10.1016/j.phrs.2010.04.002. PMID 20416377.
- Repantis, Dimitris (June 2010). "Acetylcholinesterase inhibitors and memantine for neuroenhancement in healthy individuals: A systematic review". Pharmacological Research. 61 (6): 473–481. doi:10.1016/j.phrs.2010.02.009. PMID 20193764.
- Normann, Claus; Berger, Mathias (November 2008). "Neuroenhancement: status quo and perspectives" (PDF). European Archives of Psychiatry and Clinical Neuroscience. 258: 110–114. doi:10.1007/s00406-008-5022-2. PMID 18985306. S2CID 9733191.
- Normann, Claus; Nissen, C (November 2012). "Neuroenhancement strategies for psychiatric disorders: rationale, status quo and perspectives". European Archives of Psychiatry and Clinical Neuroscience. 262: 113–116. doi:10.1007/s00406-012-0356-1. PMID 22932721. S2CID 42536705.
- Ott, R. (2012). "Neuroenhancement - perspectives of Swiss psychiatrists and general practitioners". Swiss Medical Weekly. 142: w13707. doi:10.4414/smw.2012.13707. PMID 23254869.
- Fond, Guillaume; Micoulaud-Franchi, Jean-Arthur; Brunel, Lore; Macgregor, Alexandra; Miot, Stéphanie; Lopez, Régis; Richieri, Raphaëlle; Abbar, Mocrane; Lancon, Christophe; Repantis, Dimitris (30 September 2015). "Innovative mechanisms of action for pharmaceutical cognitive enhancement: A systematic review". Psychiatry Research. 229 (1): 12–20. doi:10.1016/j.psychres.2015.07.006. ISSN 0165-1781. PMID 26187342. S2CID 23647057.
- Veit, Walter (2018). "Cognitive Enhancement and the Threat of Inequality". Journal of Cognitive Enhancement. 2 (4): 404–410. doi:10.1007/s41465-018-0108-x. S2CID 158643005.
- Franke, A. G.; Northoff, R.; Hildt, E. (November 2015). "The Case of Pharmacological Neuroenhancement: Medical, Judicial and Ethical Aspects from a German Perspective". Pharmacopsychiatry. 48 (7): 256–264. doi:10.1055/s-0035-1559640. PMID 26252723. S2CID 7179775.
- Ragan, Ian; Bard, I; Singh, I; Independent Scientific Committee on Drugs (February 2013). "What should we do about student use of cognitive enhancers? An analysis of current evidence". Neuropharmacology. 64: 588–595. doi:10.1016/j.neuropharm.2012.06.016. PMID 22732441. S2CID 207227699.
- Mereu, Maddalena; Bonci, Antonello; Newman, Amy Hauck; Tanda, Gianluigi (1 October 2013). "The neurobiology of modafinil as an enhancer of cognitive performance and a potential treatment for substance use disorders". Psychopharmacology. 229 (3): 415–434. doi:10.1007/s00213-013-3232-4. ISSN 1432-2072. PMC 3800148. PMID 23934211.
- Al-Shargie, Fares; Tariq, Usman; Mir, Hasan; Alawar, Hamad; Babiloni, Fabio; Al-Nashash, Hasan (August 2019). "Vigilance Decrement and Enhancement Techniques: A Review". Brain Sciences. 9 (8): 178. doi:10.3390/brainsci9080178. ISSN 2076-3425. PMC 6721323. PMID 31357524.
- [1][4][5][10][11][12]
- Kongkeaw, Chuenjid; Dilokthornsakul, Piyameth; Thanarangsarit, Phurit; Limpeanchob, Nanteetip; Norman Scholfield, C. (10 January 2014). "Meta-analysis of randomized controlled trials on cognitive effects of Bacopa monnieri extract". Journal of Ethnopharmacology. 151 (1): 528–535. doi:10.1016/j.jep.2013.11.008. ISSN 0378-8741. PMID 24252493.
- Aguiar, Sebastian; Borowski, Thomas (1 August 2013). "Neuropharmacological Review of the Nootropic Herb Bacopa monnieri". Rejuvenation Research. 16 (4): 313–326. doi:10.1089/rej.2013.1431. ISSN 1549-1684. PMC 3746283. PMID 23772955.
- Lorca, Cristina; Mulet, María; Arévalo-Caro, Catalina; Sanchez, M. Ángeles; Perez, Ainhoa; Perrino, María; Bach-Faig, Anna; Aguilar-Martínez, Alicia; Vilella, Elisabet; Gallart-Palau, Xavier; Serra, Aida (3 January 2022). "Plant-derived nootropics and human cognition: A systematic review". Critical Reviews in Food Science and Nutrition: 1–25. doi:10.1080/10408398.2021.2021137. PMID 34978226. S2CID 245651213.
- Kean, James D.; Downey, Luke A.; Stough, Con (December 2017). "Systematic Overview of Bacopa monnieri (L.) Wettst. Dominant Poly-Herbal Formulas in Children and Adolescents". Medicines. 4 (4): 86. doi:10.3390/medicines4040086. ISSN 2305-6320. PMC 5750610. PMID 29165401.
- Lorca, Cristina; Mulet, María; Arévalo-Caro, Catalina; Sanchez, M. Ángeles; Perez, Ainhoa; Perrino, María; Bach-Faig, Anna; Aguilar-Martínez, Alicia; Vilella, Elisabet; Gallart-Palau, Xavier; Serra, Aida (3 January 2022). "Plant-derived nootropics and human cognition: A systematic review". Critical Reviews in Food Science and Nutrition: 1–25. doi:10.1080/10408398.2021.2021137. ISSN 1040-8398. PMID 34978226. S2CID 245651213.
- [14][15][16][17][18]
- Franke, A.G.; Lieb, K. (1 August 2010). "Pharmakologisches Neuroenhancement und "Hirndoping"". Bundesgesundheitsblatt - Gesundheitsforschung - Gesundheitsschutz (in German). 53 (8): 853–860. doi:10.1007/s00103-010-1105-0. ISSN 1437-1588. PMID 20700786.
- Esposito, Massimiliano; Cocimano, Giuseppe; Ministrieri, Federica; Rosi, Giuseppe Li; Nunno, Nunzio Di; Messina, Giovanni; Sessa, Francesco; Salerno, Monica (30 August 2021). "Smart drugs and neuroenhancement: what do we know?". Frontiers in Bioscience-Landmark. 26 (8): 347–359. doi:10.52586/4948. ISSN 2768-6701. PMID 34455764.
- Losch, D.; Schulze, J. (1 November 2019). "Neuroenhancement". Zentralblatt für Arbeitsmedizin, Arbeitsschutz und Ergonomie (in German). 69 (6): 368–371. doi:10.1007/s40664-019-0340-y. ISSN 2198-0713. S2CID 240645044.
- Iglseder, Bernhard (1 February 2018). "Doping für das Gehirn". Zeitschrift für Gerontologie und Geriatrie (in German). 51 (2): 143–148. doi:10.1007/s00391-017-1351-y. ISSN 1435-1269. PMID 29209802.
- Caviola, Lucius; Faber, Nadira S. (2015). "Pills or Push-Ups? Effectiveness and Public Perception of Pharmacological and Non-Pharmacological Cognitive Enhancement". Frontiers in Psychology. 6: 1852. doi:10.3389/fpsyg.2015.01852. ISSN 1664-1078. PMC 4667098. PMID 26696922.
- Daubner, Johanna; Arshaad, Muhammad Imran; Henseler, Christina; Hescheler, Jürgen; Ehninger, Dan; Broich, Karl; Rawashdeh, Oliver; Papazoglou, Anna; Weiergräber, Marco (13 January 2021). "Pharmacological Neuroenhancement: Current Aspects of Categorization, Epidemiology, Pharmacology, Drug Development, Ethics, and Future Perspectives". Neural Plasticity. 2021: 1–27. doi:10.1155/2021/8823383. ISSN 1687-5443. PMC 7817276. PMID 33519929.
- Tang, Siu W.; Tang, Wayne H.; Leonard, Brian E. (July 2017). "Managing interactions between cognitive enhancers and other psychotropics". International Clinical Psychopharmacology. 32 (4): 175–183. doi:10.1097/YIC.0000000000000172. PMID 28234656. S2CID 4024803.
- [20][9][12][21][22][16][23][24][25][26]
- Jangwan, Nitish Singh; Ashraf, Ghulam Md; Ram, Veerma; Singh, Vinod; Alghamdi, Badrah S.; Abuzenadah, Adel Mohammad; Singh, Mamta F. (2022). "Brain augmentation and neuroscience technologies: current applications, challenges, ethics and future prospects". Frontiers in Systems Neuroscience. 16. doi:10.3389/fnsys.2022.1000495. PMC 9538357. PMID 36211589.
- Nair, Prashant (2013-11-12). "Brain–machine interface". Proceedings of the National Academy of Sciences. 110 (46): 18343. Bibcode:2013PNAS..11018343N. doi:10.1073/pnas.1319310110. ISSN 0027-8424. PMC 3831969. PMID 24222678.
- ""nootropic"[Title/Abstract] OR "smart drug"[Title/Abstract] - Search Results - PubMed". PubMed. Retrieved 20 March 2023.
- Wood, Suzanne; Sage, Jennifer R.; Shuman, Tristan; Anagnostaras, Stephan G. (1 January 2014). "Psychostimulants and Cognition: A Continuum of Behavioral and Cognitive Activation". Pharmacological Reviews. 66 (1): 193–221. doi:10.1124/pr.112.007054. ISSN 0031-6997. PMC 3880463. PMID 24344115.
- Schifano, Fabrizio; Catalani, Valeria; Sharif, Safia; Napoletano, Flavia; Corkery, John Martin; Arillotta, Davide; Fergus, Suzanne; Vento, Alessandro; Guirguis, Amira (1 April 2022). "Benefits and Harms of 'Smart Drugs' (Nootropics) in Healthy Individuals". Drugs. 82 (6): 633–647. doi:10.1007/s40265-022-01701-7. ISSN 1179-1950. PMID 35366192. S2CID 247860331.
- Dresler, Martin; Sandberg, Anders; Bublitz, Christoph; Ohla, Kathrin; Trenado, Carlos; Mroczko-Wąsowicz, Aleksandra; Kühn, Simone; Repantis, Dimitris (20 March 2019). "Hacking the Brain: Dimensions of Cognitive Enhancement". ACS Chemical Neuroscience. 10 (3): 1137–1148. doi:10.1021/acschemneuro.8b00571. ISSN 1948-7193. PMC 6429408. PMID 30550256.
- Wingelaar-Jagt, Yara Q.; Bottenheft, Charelle; Riedel, Wim J.; Ramaekers, Johannes G. (February 2023). "Effects of modafinil and caffeine on night-time vigilance of air force crewmembers: A randomized controlled trial". Journal of Psychopharmacology. 37 (2): 172–180. doi:10.1177/02698811221142568. ISSN 0269-8811. PMC 9912306. PMID 36515156.
- Graf, William D.; Nagel, Saskia K.; Epstein, Leon G.; Miller, Geoffrey; Nass, Ruth; Larriviere, Dan (26 March 2013). "Pediatric neuroenhancement: Ethical, legal, social, and neurodevelopmental implications". Neurology. 80 (13): 1251–1260. doi:10.1212/WNL.0b013e318289703b. ISSN 0028-3878. PMID 23486879. S2CID 207122859.
- Weiergräber, Marco; Ehninger, Dan; Broich, Karl (1 April 2017). "Neuroenhancement and mood enhancement – Physiological and pharmacodynamical background". Medizinische Monatsschrift für Pharmazeuten. 40 (4): 154–164. ISSN 0342-9601. PMID 29952165.
- Marazziti, Donatella; Avella, Maria Teresa; Ivaldi, Tea; Palermo, Stefania; Massa, Lucia; Della Vecchia, Alessandra; Basile, Lucia; Mucci, Federico (June 2021). "Neuroenhancement: state of the art and future perspectives". Clinical Neuropsychiatry. 18 (3): 137–169. doi:10.36131/cnfioritieditore20210303. PMC 8629054. PMID 34909030.
- d'Angelo, L-S Camilla; Savulich, George; Sahakian, Barbara J (October 2017). "Lifestyle use of drugs by healthy people for enhancing cognition, creativity, motivation and pleasure: Lifestyle use of drugs by healthy people". British Journal of Pharmacology. 174 (19): 3257–3267. doi:10.1111/bph.13813. PMC 5595759. PMID 28427114.
- Brühl, Annette B.; d'Angelo, Camilla; Sahakian, Barbara J. (January 2019). "Neuroethical issues in cognitive enhancement: Modafinil as the example of a workplace drug?". Brain and Neuroscience Advances. 3: 239821281881601. doi:10.1177/2398212818816018. PMC 7058249. PMID 32166175.
- Bornemann, Joel (7 August 2020). "The Viability of Microdosing Psychedelics as a Strategy to Enhance Cognition and Well-being - An Early Review". Journal of Psychoactive Drugs. 52 (4): 300–308. doi:10.1080/02791072.2020.1761573. ISSN 0279-1072. PMID 32362269. S2CID 218493319.
- de Jongh, Reinoud; Bolt, Ineke; Schermer, Maartje; Olivier, Berend (1 January 2008). "Botox for the brain: enhancement of cognition, mood and pro-social behavior and blunting of unwanted memories". Neuroscience & Biobehavioral Reviews. 32 (4): 760–776. doi:10.1016/j.neubiorev.2007.12.001. ISSN 0149-7634. PMID 18295885. S2CID 7252617.
- Sahakian, Barbara J.; Bruhl, Annette B.; Cook, Jennifer; Killikelly, Clare; Savulich, George; Piercy, Thomas; Hafizi, Sepehr; Perez, Jesus; Fernandez-Egea, Emilio; Suckling, John; Jones, Peter B. (19 September 2015). "The impact of neuroscience on society: cognitive enhancement in neuropsychiatric disorders and in healthy people". Philosophical Transactions of the Royal Society B: Biological Sciences. 370 (1677): 20140214. doi:10.1098/rstb.2014.0214. ISSN 0962-8436. PMC 4528826. PMID 26240429.
- Brühl, Annette B.; Sahakian, Barbara J. (2016). "Drugs, games, and devices for enhancing cognition: implications for work and society". Annals of the New York Academy of Sciences. 1369 (1): 195–217. Bibcode:2016NYASA1369..195B. doi:10.1111/nyas.13040. PMID 27043232. S2CID 5111793.
- Burk, Joshua A.; Blumenthal, Sarah A.; Maness, Eden B. (15 September 2018). "Neuropharmacology of attention". European Journal of Pharmacology. 835: 162–168. doi:10.1016/j.ejphar.2018.08.008. ISSN 0014-2999. PMC 6140347. PMID 30092180.
- Tuenter, Emmy; Foubert, Kenn; Pieters, Luc (August 2018). "Mood Components in Cocoa and Chocolate: The Mood Pyramid". Planta Medica. 84 (12/13): 839–844. doi:10.1055/a-0588-5534. ISSN 0032-0943. PMID 29539647. S2CID 3912460.
- Marcora, Samuele (1 January 2016). "Can Doping be a Good Thing? Using Psychoactive Drugs to Facilitate Physical Activity Behaviour". Sports Medicine. 46 (1): 1–5. doi:10.1007/s40279-015-0412-x. ISSN 1179-2035. PMID 26497149. S2CID 13448073.
- Porsdam Mann, Sebastian; Sahakian, Barbara J (1 August 2015). "The increasing lifestyle use of modafinil by healthy people: safety and ethical issues". Current Opinion in Behavioral Sciences. 4: 136–141. doi:10.1016/j.cobeha.2015.05.004. ISSN 2352-1546. S2CID 53198934.
- Mun, Monique; Wong, Andrew (14 December 2020). "Kratom and Phenibut: A Concise Review for Psychiatric Trainees". American Journal of Psychiatry Residents' Journal. 16 (2): 6–8. doi:10.1176/appi.ajp-rj.2020.160203. ISSN 2474-4662. S2CID 230589322.
- Peele, Stanton; Brodsky, Archie (10 November 2000). "Exploring psychological benefits associated with moderate alcohol use: a necessary corrective to assessments of drinking outcomes?". Drug and Alcohol Dependence. 60 (3): 221–247. doi:10.1016/S0376-8716(00)00112-5. ISSN 0376-8716. PMID 11053757.
- Wudarczyk, Olga A.; Earp, Brian D.; Guastella, Adam; Savulescu, Julian (September 2013). "Could intranasal oxytocin be used to enhance relationships? Research imperatives, clinical policy, and ethical considerations". Current Opinion in Psychiatry. 26 (5): 474–484. doi:10.1097/YCO.0b013e3283642e10. PMC 3935449. PMID 23880593.
- Buckner, Julia D.; Morris, Paige E.; Abarno, Cristina N.; Glover, Nina I.; Lewis, Elizabeth M. (17 April 2021). "Biopsychosocial Model Social Anxiety and Substance Use Revised". Current Psychiatry Reports. 23 (6): 35. doi:10.1007/s11920-021-01249-5. ISSN 1535-1645. PMID 33864136. S2CID 233261493.
- Peltier, MacKenzie R.; Sofuoglu, Mehmet (1 January 2020). "Chapter 23 - Pharmacological cognitive enhancers". Cognition and Addiction. Academic Press. pp. 303–320. ISBN 978-0-12-815298-0.
- Brady, Kathleen T.; Gray, Kevin M.; Tolliver, Bryan K. (1 August 2011). "Cognitive enhancers in the treatment of substance use disorders: Clinical evidence". Pharmacology Biochemistry and Behavior. 99 (2): 285–294. doi:10.1016/j.pbb.2011.04.017. ISSN 0091-3057. PMC 3114106. PMID 21557964.
- Kampman, Kyle M. (4 October 2019). "The treatment of cocaine use disorder". Science Advances. 5 (10): eaax1532. Bibcode:2019SciA....5.1532K. doi:10.1126/sciadv.aax1532. ISSN 2375-2548. PMC 6795516. PMID 31663022.
- Fluyau, Dimy; Cook, Sarah Clare; Chima, Ashmeer; Kailasam, Vasanth Kattalai; Revadigar, Neelambika (1 November 2021). "Pharmacological management of psychoactive substance withdrawal syndrome". Drugs & Therapy Perspectives. 37 (11): 519–535. doi:10.1007/s40267-021-00874-7. ISSN 1179-1977. S2CID 244583239.
- Erler, Alexandre; Forlini, Cynthia (2020). "Neuroenhancement". Routledge Encyclopedia of Philosophy Online. Retrieved 9 March 2023.
- Beda, Zsolt; Smith, Steven M.; Orr, Joseph (1 August 2020). "Creativity on demand – Hacking into creative problem solving". NeuroImage. 216: 116867. doi:10.1016/j.neuroimage.2020.116867. ISSN 1053-8119. PMID 32325208. S2CID 215823256.
- Lanni, Cristina; Lenzken, Silvia C.; Pascale, Alessia; Del Vecchio, Igor; Racchi, Marco; Pistoia, Francesca; Govoni, Stefano (1 March 2008). "Cognition enhancers between treating and doping the mind". Pharmacological Research. 57 (3): 196–213. doi:10.1016/j.phrs.2008.02.004. ISSN 1043-6618. PMID 18353672.
- Tennison, Michael N.; Moreno, Jonathan D. (2017). "Neuroenhancement and Therapy in National Defense Contexts". The Routledge Handbook of Neuroethics. pp. 150–165. doi:10.4324/9781315708652-12. ISBN 978-1-315-70865-2.
- "Neuroenhancement in Military Personnel: Conceptual and Methodological Promises and Challenge". Retrieved 10 March 2023.
- Crawford, Cindy; Teo, Lynn; Lafferty, Lynn; Drake, Angela; Bingham, John J.; Gallon, Matthew D.; O'Connell, Meghan L.; Chittum, Holly K.; Arzola, Sonya M.; Berry, Kevin (June 2017). "Caffeine to optimize cognitive function for military mission-readiness: a systematic review and recommendations for the field". Nutrition Reviews. 75 (suppl_2): 17–35. doi:10.1093/nutrit/nux007. PMID 28969341.
- Bagot, Kara Simone; Kaminer, Yifrah (2014). "Efficacy of stimulants for cognitive enhancement in non-attention deficit hyperactivity disorder youth: a systematic review". Addiction. 109 (4): 547–557. doi:10.1111/add.12460. PMC 4471173. PMID 24749160.
- Mahdiani, Hamideh; Ungar, Michael (August 2021). "Can biomedical and cognitive enhancement increase psychological resilience?". Canadian Psychology. 62 (3): 295–303. doi:10.1037/cap0000217. ISSN 1878-7304. S2CID 216387797.
- Budde, Henning; Wegner, Mirko (17 April 2018). The Exercise Effect on Mental Health: Neurobiological Mechanisms. CRC Press. ISBN 978-1-4987-3953-5.
- Sherman, Brian J.; McRae-Clark, Aimee L. (May 2016). "Treatment of Cannabis Use Disorder: Current Science and Future Outlook". Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy. 36 (5): 511–535. doi:10.1002/phar.1747. PMC 4880536. PMID 27027272.
- Bortolato, Beatrice; Miskowiak, Kamilla W.; Köhler, Cristiano A.; Maes, Michael; Fernandes, Brisa S.; Berk, Michael; Carvalho, André F. (22 January 2016). "Cognitive remission: a novel objective for the treatment of major depression?". BMC Medicine. 14 (1): 9. doi:10.1186/s12916-016-0560-3. ISSN 1741-7015. PMC 4724131. PMID 26801406.
- Ooi, Soo Liang; Green, Ruth; Pak, Sok Cheon (22 October 2018). "N-Acetylcysteine for the Treatment of Psychiatric Disorders: A Review of Current Evidence". BioMed Research International. 2018: e2469486. doi:10.1155/2018/2469486. ISSN 2314-6133. PMC 6217900. PMID 30426004.
- Nery, Fabiano G.; Li, Wenbin; DelBello, Melissa P.; Welge, Jeffrey A. (15 January 2021). "N-acetylcysteine as an adjunctive treatment for bipolar depression: A systematic review and meta-analysis of randomized controlled trials". Bipolar Disorders. 23 (7): 707–714. doi:10.1111/bdi.13039. ISSN 1398-5647. PMID 33354859. S2CID 229692736.
- Colpo, Gabriela D.; Leboyer, Marion; Dantzer, Robert; Trivedi, Mahdukar H.; Teixeira, Antonio L. (1 February 2018). "Immune-based strategies for mood disorders: facts and challenges". Expert Review of Neurotherapeutics. 18 (2): 139–152. doi:10.1080/14737175.2018.1407242. ISSN 1473-7175. PMC 5819337. PMID 29179585.
- Fernandes, Brisa S.; Dean, Olivia M.; Dodd, Seetal; Malhi, Gin S.; Berk, Michael (27 April 2016). "N-Acetylcysteine in Depressive Symptoms and Functionality: A Systematic Review and Meta-Analysis". The Journal of Clinical Psychiatry. 77 (4): e457-66. doi:10.4088/JCP.15r09984. ISSN 0160-6689. PMID 27137430. S2CID 40688371.
- ""Modafinil"[Title/Abstract] - Search Results - PubMed". PubMed. Retrieved 13 March 2023.
- Woolf, Nicky (31 May 2015). "Silk Road sentencing: why governments can't win the war on darknet drugs". The Guardian. Retrieved 30 December 2016.
- Valette, Jean-Jacques (9 September 2016). "Du commerce illicite à la liberté d'expression totale, on a plongé dans le darknet". We Demain. Retrieved 30 December 2016.
- "("enhance"[Title/Abstract] AND "cognition"[Title/Abstract]) OR ("neuroenhancement"[Title/Abstract]) OR ("cognitive enhancement"[Title/Abstract]) - Search Results - PubMed". PubMed. Retrieved 13 March 2023.
- Hara, Y.; McKeehan, N.; Dacks, P. A.; Fillit, H. M. (1 September 2017). "Evaluation of the neuroprotective potential of n-acetylcysteine for prevention and treatment of cognitive aging and dementia". Journal of Prevention of Alzheimer's Disease. J Prev Alz Dis 20174 (3): 201–206. doi:10.14283/jpad.2017.22. PMID 29182711. S2CID 45647979.
- Zhao, Danyue; Simon, James E.; Wu, Qingli (21 February 2020). "A critical review on grape polyphenols for neuroprotection: Strategies to enhance bioefficacy". Critical Reviews in Food Science and Nutrition. 60 (4): 597–625. doi:10.1080/10408398.2018.1546668. ISSN 1040-8398. PMID 30614258. S2CID 58598405.
- Onaolapo, Adejoke Yetunde; Obelawo, Adebimpe Yemisi; Onaolapo, Olakunle James (2019). "Brain Ageing, Cognition and Diet: A Review of the Emerging Roles of Food-Based Nootropics in Mitigating Age-related Memory Decline". Current Aging Science. 12 (1): 2–14. doi:10.2174/1874609812666190311160754. PMC 6971896. PMID 30864515.
- Ali, Fahad; Naz, Falaq; Jyoti, Smita; Siddique, Yasir Hasan (3 June 2017). "Health functionality of apigenin: A review". International Journal of Food Properties. 20 (6): 1197–1238. doi:10.1080/10942912.2016.1207188. ISSN 1094-2912. S2CID 88642353.
- Bonferoni, Maria Cristina; Rossi, Silvia; Sandri, Giuseppina; Ferrari, Franca; Gavini, Elisabetta; Rassu, Giovanna; Giunchedi, Paolo (February 2019). "Nanoemulsions for "Nose-to-Brain" Drug Delivery". Pharmaceutics. 11 (2): 84. doi:10.3390/pharmaceutics11020084. ISSN 1999-4923. PMC 6409749. PMID 30781585.
- Kaurav, Hemlata; Kapoor, Deepak N (December 2017). "Implantable systems for drug delivery to the brain". Therapeutic Delivery. 8 (12): 1097–1107. doi:10.4155/tde-2017-0082. PMID 29125063.
- Darwish, Mona; Kirby, Mary; Hellriegel, Edward T.; Robertson, Philmore (1 September 2009). "Armodafinil and Modafinil Have Substantially Different Pharmacokinetic Profiles Despite Having the Same Terminal Half-Lives". Clinical Drug Investigation. 29 (9): 613–623. doi:10.2165/11315280-000000000-00000. ISSN 1179-1918. PMID 19663523. S2CID 6607186.
- Sheng, Ping; Hou, Lijun; Wang, Xiang; Wang, Xiaowen; Huang, Chengguang; Yu, Mingkun; Han, Xi; Dong, Yan (3 December 2013). "Efficacy of Modafinil on Fatigue and Excessive Daytime Sleepiness Associated with Neurological Disorders: A Systematic Review and Meta-Analysis". PLOS ONE. 8 (12): e81802. Bibcode:2013PLoSO...881802S. doi:10.1371/journal.pone.0081802. PMC 3849275. PMID 24312590.
- Darwish, Mona; Kirby, Mary; Hellriegel, Edward T.; Yang, Ronghua; Robertson, Philmore (1 February 2009). "Pharmacokinetic Profile of Armodafinil in Healthy Subjects". Clinical Drug Investigation. 29 (2): 87–100. doi:10.2165/0044011-200929020-00003. ISSN 1179-1918. PMID 19133704. S2CID 24886727.
- "Getting smart to cognitive enhancers". eClinicalMedicine. 11: 1–2. 1 May 2019. doi:10.1016/j.eclinm.2019.06.014. ISSN 2589-5370. PMC 6610764. PMID 31312801. S2CID 196810318.
- Malík, Matěj; Tlustoš, Pavel (January 2022). "Nootropics as Cognitive Enhancers: Types, Dosage and Side Effects of Smart Drugs". Nutrients. 14 (16): 3367. doi:10.3390/nu14163367. ISSN 2072-6643. PMC 9415189. PMID 36014874.
- Lynch, Gary (2014). "Pharmacological enhancement of memory or cognition in normal subjects". Frontiers in Systems Neuroscience. 8: 90. doi:10.3389/fnsys.2014.00090. PMC 4033242. PMID 24904313.
- Fernstrom, John D.; Fernstrom, Madelyn H. (1 June 2007). "Tyrosine, Phenylalanine, and Catecholamine Synthesis and Function in the Brain". The Journal of Nutrition. 137 (6): S1539–S1547. doi:10.1093/jn/137.6.1539S. ISSN 0022-3166. PMID 17513421.
- Riedel, Wim J; Klaassen, Tineke; Schmitt, Jeroen A. J (1 October 2002). "Tryptophan, mood, and cognitive function". Brain, Behavior, and Immunity. 16 (5): 581–589. doi:10.1016/S0889-1591(02)00013-2. ISSN 0889-1591. PMID 12401472. S2CID 42931109.
[...] As noted above two experiments addressed the question how [acute tryptophan depletion (ATD)] affects cognitive functions in healthy individuals. These experiments shared one common dependent variable, which was the memory task. In the first study it was shown that ATD impaired long-term memory consolidation when a new word list was learned at 6 h after start of depletion [...]
- Camfield, David A; Stough, Con; Farrimond, Jonathon; Scholey, Andrew B (August 2014). "Acute effects of tea constituents L-theanine, caffeine, and epigallocatechin gallate on cognitive function and mood: a systematic review and meta-analysis". Nutrition Reviews. 72 (8): 507–522. doi:10.1111/nure.12120. PMID 24946991.
- Sohail, Anas Anas; Ortiz, Fernando; Varghese, Teresa; Fabara, Stephanie P.; Batth, Arshdeep S.; Sandesara, Darshan P.; Sabir, Ahtesham; Khurana, Mahika; Datta, Shae; Patel, Urvish K.; Sohail, Anas Anas; Ortiz, Juan Fernando; Varghese, Teresa; Fabara, Stephanie P.; Batth, Arshdeep S.; Sandesara, Darshan P.; Sabir, Ahtesham; Khurana, Mahika; Datta, Shae; Patel, Urvish K. (30 December 2021). "The Cognitive-Enhancing Outcomes of Caffeine and L-theanine: A Systematic Review". Cureus. 13 (12): e20828. doi:10.7759/cureus.20828. ISSN 2168-8184. PMC 8794723. PMID 35111479.
- Meeusen, Romain; Decroix, Lieselot (1 March 2018). "Nutritional Supplements and the Brain". International Journal of Sport Nutrition and Exercise Metabolism. 28 (2): 200–211. doi:10.1123/ijsnem.2017-0314. ISSN 1543-2742. PMID 29252056. S2CID 4582295.
- Gunzelmann, Glenn; M. James, Stephen; Caldwell, Jo Lynn (1 January 2019). "Basic and applied science interactions in fatigue understanding and risk mitigation". Chapter 8 - Basic and applied science interactions in fatigue understanding and risk mitigation. Progress in Brain Research. Vol. 246. Elsevier. pp. 177–204. doi:10.1016/bs.pbr.2019.03.022. ISBN 978-0-444-64250-9. PMID 31072561. S2CID 145885926.
- Young, Allan H (June 2021). "'Memory, hither come': Psychopharmacology of memory and more". Journal of Psychopharmacology. 35 (6): 619–620. doi:10.1177/02698811211021065. ISSN 0269-8811. PMID 34039101. S2CID 235217295.
- Gonzalez, Natalie A.; Sakhamuri, Navya; Athiyaman, Sreekartthik; Randhi, Bhawna; Gutlapalli, Sai Dheeraj; Pu, Jingxiong; Zaidi, Maheen F.; Patel, Maithily; Atluri, Lakshmi Malvika; Franchini, Ana P. Arcia; Gonzalez, Natalie A.; Sakhamuri, Navya; Athiyaman, Sreekartthik; Randhi, Bhawna; Gutlapalli, Sai Dheeraj; Pu, Jingxiong; Zaidi, Maheen F.; Patel, Maithily; Atluri, Lakshmi Malvika; Franchini, Ana P. Arcia (14 March 2023). "A Systematic Review of Yoga and Meditation for Attention-Deficit/Hyperactivity Disorder in Children". Cureus. 15 (3): e36143. doi:10.7759/cureus.36143. ISSN 2168-8184. PMC 10101238. PMID 37065343. S2CID 257541572.
- Baumel, Barry S.; Doraiswamy, P. Murali; Sabbagh, Marwan; Wurtman, Richard (1 June 2021). "Potential Neuroregenerative and Neuroprotective Effects of Uridine/Choline-Enriched Multinutrient Dietary Intervention for Mild Cognitive Impairment: A Narrative Review". Neurology and Therapy. 10 (1): 43–60. doi:10.1007/s40120-020-00227-y. ISSN 2193-6536. PMC 8139993. PMID 33368017.
- Wurtman, Richard J; Cansev, Mehmet; Sakamoto, Toshimasa; Ulus, Ismael (December 2010). "Nutritional modifiers of aging brain function: use of uridine and other phosphatide precursors to increase formation of brain synapses: Nutrition Reviews, Vol. 68, No. s2". Nutrition Reviews. 68 (Suppl 2): S88–S101. doi:10.1111/j.1753-4887.2010.00344.x. PMC 3062998. PMID 21091953.
- Cardoso, Carlos; Afonso, Cláudia; Bandarra, Narcisa M. (December 2016). "Dietary DHA and health: cognitive function ageing". Nutrition Research Reviews. 29 (2): 281–294. doi:10.1017/S0954422416000184. ISSN 0954-4224. PMID 27866493. S2CID 4243219.
- Van Puyvelde, Martine; Van Cutsem, Jeroen; Lacroix, Emilie; Pattyn, Nathalie (11 October 2021). "A State-of-the-Art Review on the Use of Modafinil as A Performance-enhancing Drug in the Context of Military Operationality". Military Medicine. 187 (11–12): 1286–1298. doi:10.1093/milmed/usab398. ISSN 0026-4075. PMID 34632515.
- Mohamed, Ahmed Dahir (September 2014). "Neuroethical issues in pharmacological cognitive enhancement". WIREs Cognitive Science. 5 (5): 533–549. doi:10.1002/wcs.1306. ISSN 1939-5078. PMID 26308743.
- Sousa, Ana; Dinis-Oliveira, Ricardo Jorge (2 April 2020). "Pharmacokinetic and pharmacodynamic of the cognitive enhancer modafinil: Relevant clinical and forensic aspects". Substance Abuse. 41 (2): 155–173. doi:10.1080/08897077.2019.1700584. ISSN 0889-7077. PMID 31951804. S2CID 210709160.
- Minzenberg, Michael J; Carter, Cameron S (June 2008). "Modafinil: A Review of Neurochemical Actions and Effects on Cognition". Neuropsychopharmacology. 33 (7): 1477–1502. doi:10.1038/sj.npp.1301534. PMID 17712350. S2CID 13752498.
- Hofmann, Stefan G.; Mundy, Elizabeth A.; Curtiss, Joshua; Hofmann, Stefan G.; Mundy, Elizabeth A.; Curtiss, Joshua (2015). "Neuroenhancement of Exposure Therapy in Anxiety Disorders". AIMS Neuroscience. 2 (3): 123–138. doi:10.3934/Neuroscience.2015.3.123. PMC 4545667. PMID 26306326.
- Zager, Adriano (1 August 2020). "Modulating the immune response with the wake-promoting drug modafinil: A potential therapeutic approach for inflammatory disorders". Brain, Behavior, and Immunity. 88: 878–886. doi:10.1016/j.bbi.2020.04.038. ISSN 0889-1591. PMID 32311496. S2CID 215807973.
- Müller, Christian P. (15 July 2020). "Drug instrumentalization". Behavioural Brain Research. 390: 112672. doi:10.1016/j.bbr.2020.112672. ISSN 0166-4328. PMID 32442549. S2CID 218710837.
- Heilman, Kenneth M. (June 2016). "Possible Brain Mechanisms of Creativity". Archives of Clinical Neuropsychology. 31 (4): 285–296. doi:10.1093/arclin/acw009. PMID 27001974.
- Lyon, Regan F; Gramm, Joshua; Branagan, Brian; Houck, Shannon C (2022). "Implications of Neurological Directed-Energy Weapons for Military Medicine". Journal of Special Operations Medicine. 22 (3): 104–107. doi:10.55460/0jal-jijt. PMID 35877979. S2CID 251067896.
- Johnson, Robert A. (1 March 2014). "Predicting Future War". The US Army War College Quarterly: Parameters. 44 (1). doi:10.55540/0031-1723.2801. ISSN 0031-1723. S2CID 89604319.
- Turner, Grant (2021). "The Unspoken Arms Race: Neurowarfare". SSRN Electronic Journal. doi:10.2139/ssrn.3943670. S2CID 239024129.
- Gramm, Joshua D.; Branagan, Brian A. (2021-12-01). "Neurowar is Here!". Retrieved 20 March 2023.
- White, Stephen (1 January 2008). "Brave New World: Neurowarfare and the Limits of International Humanitarian Law". Cornell International Law Journal. 41 (1): 177–210. ISSN 0010-8812.
- Krishnan, Armin (4 October 2016). Military Neuroscience and the Coming Age of Neurowarfare. Taylor & Francis. ISBN 978-1-317-09607-8. Retrieved 20 March 2023.
- "Weapons of perception: neuroscience and mind-controlled weapons". Army Technology. 21 May 2012. Retrieved 20 March 2023.
- Belous, Anatoly; Saladukha, Vitali (2020). "Modern Weapons: Possibilities and Limitations". Viruses, Hardware and Software Trojans: Attacks and Countermeasures. Springer International Publishing. pp. 731–820. ISBN 978-3-030-47218-4.
- Drollette, Dan (5 October 2016). "When neuroscience leads to neuroweapons". Bulletin of the Atomic Scientists. Retrieved 20 March 2023.
- Diggins, Chloe. "Hacking the Human Brain: The Next Domain of Warfare". Wired. Retrieved 20 March 2023.
- "Neuroscience the new face of warfare: experts". Reuters. 7 February 2012. Retrieved 20 March 2023.
- Kerick, Scott E. (2022-03-01). "Literature Review on Human Bioeffects of Electromagnetic Energy: A Complex Systems Perspective". Retrieved 21 March 2023.
- "Weaponizing the Brain: Neuroscience Advancements Spark Debate". www.nationaldefensemagazine.org. Retrieved 20 March 2023.
- Tennison, Michael N.; Moreno, Jonathan D. (20 March 2012). "Neuroscience, Ethics, and National Security: The State of the Art". PLOS Biology. 10 (3): e1001289. doi:10.1371/journal.pbio.1001289. ISSN 1545-7885. PMC 3308927. PMID 22448146.
- Ngo, Thao; Ghio, Marta; Kuchinke, Lars; Roser, Patrik; Bellebaum, Christian (1 September 2019). "Moral decision making under modafinil: a randomized placebo-controlled double-blind crossover fMRI study". Psychopharmacology. 236 (9): 2747–2759. doi:10.1007/s00213-019-05250-y. ISSN 1432-2072. PMID 31037409. S2CID 253751563.
- Earp, Brian D.; Douglas, Thomas; Savulescu, Julian (2017). "Moral Neuroenhancement". The Routledge Handbook of Neuroethics. Routledge. ISBN 978-1-138-89829-5.
- Mann, Sebastian Porsdam; Sahakian, Barbara J. "Modafinil and the Increasing Lifestyle Use of Smart Drugs by Healthy People: Neuroethical and Societal Issues". The Routledge Handbook of Neuroethics.
- Scarmeas, Nikolaos; Anastasiou, Costas A.; Yannakoulia, Mary (1 November 2018). "Nutrition and prevention of cognitive impairment" (PDF). The Lancet Neurology. 17 (11): 1006–1015. doi:10.1016/S1474-4422(18)30338-7. ISSN 1474-4422. PMID 30244829. S2CID 52347029.
- Wonodi, Ikwunga; Schwarcz, Robert (March 2010). "Cortical kynurenine pathway metabolism: a novel target for cognitive enhancement in Schizophrenia". Schizophrenia Bulletin. 36 (2): 211–218. doi:10.1093/schbul/sbq002. ISSN 1745-1701. PMC 2833131. PMID 20147364.
- Morè, Lorenzo; Lauterborn, Julie C.; Papaleo, Francesco; Brambilla, Riccardo (March 2020). "Enhancing cognition through pharmacological and environmental interventions: Examples from preclinical models of neurodevelopmental disorders" (PDF). Neuroscience and Biobehavioral Reviews. 110: 28–45. doi:10.1016/j.neubiorev.2019.02.003. ISSN 1873-7528. PMID 30981451. S2CID 86554761.
- Tennison, Michael N.; Moreno, Jonathan D. "Neuroenhancement and Therapy in National Defense Contexts". The Routledge Handbook of Neuroethics.
- "Genetically modified neurons could help us connect to implants". New Scientist. Retrieved 1 February 2022.
- "Scientists program cells to carry out gene-guided construction projects". phys.org. Retrieved 5 April 2020.
- Otto, Kevin J.; Schmidt, Christine E. (20 March 2020). "Neuron-targeted electrical modulation". Science. 367 (6484): 1303–1304. Bibcode:2020Sci...367.1303O. doi:10.1126/science.abb0216. PMID 32193309. S2CID 213192749.
- Liu, Jia; Kim, Yoon Seok; Richardson, Claire E.; Tom, Ariane; Ramakrishnan, Charu; Birey, Fikri; Katsumata, Toru; Chen, Shucheng; Wang, Cheng; Wang, Xiao; Joubert, Lydia-Marie; Jiang, Yuanwen; Wang, Huiliang; Fenno, Lief E.; Tok, Jeffrey B.-H.; Pașca, Sergiu P.; Shen, Kang; Bao, Zhenan; Deisseroth, Karl (20 March 2020). "Genetically targeted chemical assembly of functional materials in living cells, tissues, and animals". Science. 367 (6484): 1372–1376. Bibcode:2020Sci...367.1372L. doi:10.1126/science.aay4866. PMC 7527276. PMID 32193327. S2CID 213191980.
- Bolakhe, Saugat. "Lego Robot with an Organic 'Brain' Learns to Navigate a Maze". Scientific American. Retrieved 1 February 2022.
- Sample, Ian (3 December 2019). "Bionic neurons could enable implants to restore failing brain circuits". The Guardian. Retrieved 27 February 2023.
- Abu-Hassan, Kamal; Taylor, Joseph D.; Morris, Paul G.; Donati, Elisa; Bortolotto, Zuner A.; Indiveri, Giacomo; Paton, Julian F. R.; Nogaret, Alain (3 December 2019). "Optimal solid state neurons". Nature Communications. 10 (1): 5309. Bibcode:2019NatCo..10.5309A. doi:10.1038/s41467-019-13177-3. ISSN 2041-1723. PMC 6890780. PMID 31796727.
- Buchanan, Allen (July 2011). "Cognitive enhancement and education". Theory and Research in Education. 9 (2): 145–162. doi:10.1177/1477878511409623. ISSN 1477-8785. S2CID 145441073.
- Nakhaee, Samaneh; Kooshki, Alireza; Hormozi, Ali; Akbari, Aref; Mehrpour, Omid; Farrokhfall, Khadijeh (18 January 2023). "Cinnamon and cognitive function: a systematic review of preclinical and clinical studies". Nutritional Neuroscience: 1–15. doi:10.1080/1028415X.2023.2166436. ISSN 1028-415X. PMID 36652384. S2CID 255969320.
- Gratton, Gabriele; Weaver, Samuel R.; Burley, Claire V.; Low, Kathy A.; Maclin, Edward L.; Johns, Paul W.; Pham, Quang S.; Lucas, Samuel J. E.; Fabiani, Monica; Rendeiro, Catarina (24 November 2020). "Dietary flavanols improve cerebral cortical oxygenation and cognition in healthy adults". Scientific Reports. 10 (1): 19409. Bibcode:2020NatSR..1019409G. doi:10.1038/s41598-020-76160-9. ISSN 2045-2322. PMC 7687895. PMID 33235219.
- Melzer, Thayza Martins; Manosso, Luana Meller; Yau, Suk-yu; Gil-Mohapel, Joana; Brocardo, Patricia S. (January 2021). "In Pursuit of Healthy Aging: Effects of Nutrition on Brain Function". International Journal of Molecular Sciences. 22 (9): 5026. doi:10.3390/ijms22095026. ISSN 1422-0067. PMC 8126018. PMID 34068525.
- Socci, Valentina; Tempesta, Daniela; Desideri, Giovambattista; De Gennaro, Luigi; Ferrara, Michele (2017). "Enhancing Human Cognition with Cocoa Flavonoids". Frontiers in Nutrition. 4: 19. doi:10.3389/fnut.2017.00019. ISSN 2296-861X. PMC 5432604. PMID 28560212.
- Kent, K.; Charlton, K. E.; Netzel, M.; Fanning, K. (June 2017). "Food-based anthocyanin intake and cognitive outcomes in human intervention trials: a systematic review". Journal of Human Nutrition and Dietetics. 30 (3): 260–274. doi:10.1111/jhn.12431. PMID 27730693. S2CID 4344504.
- Zamri, Nurul Ashykin; Ghani, Nurhafizah; Ismail, Che Aishah Nazariah; Zakaria, Rahimah; Shafin, Nazlahshaniza (2023). "Honey on brain health: A promising brain booster". Frontiers in Aging Neuroscience. 14. doi:10.3389/fnagi.2022.1092596. ISSN 1663-4365. PMC 9887050. PMID 36733498.
- Kennedy, David O. (1 September 2014). "Polyphenols and the Human Brain: Plant "Secondary Metabolite" Ecologic Roles and Endogenous Signaling Functions Drive Benefits". Advances in Nutrition. 5 (5): 515–533. doi:10.3945/an.114.006320. ISSN 2161-8313. PMC 4188223. PMID 25469384.
- Gomez-Pinilla, Fernando; Nguyen, Trang T J (1 May 2012). "Natural mood foods: The actions of polyphenols against psychiatric and cognitive disorders". Nutritional Neuroscience. 15 (3): 127–133. doi:10.1179/1476830511Y.0000000035. ISSN 1028-415X. PMC 3355196. PMID 22334236.
- Vauzour, David; Rodriguez-Mateos, Ana; Corona, Giulia; Oruna-Concha, Maria Jose; Spencer, Jeremy P. E. (November 2010). "Polyphenols and Human Health: Prevention of Disease and Mechanisms of Action". Nutrients. 2 (11): 1106–1131. doi:10.3390/nu2111106. ISSN 2072-6643. PMC 3257622. PMID 22254000.
- Guerrieri, Davide; Moon, Hyo Youl; van Praag, Henriette (1 January 2017). "Exercise in a Pill: The Latest on Exercise-Mimetics". Brain Plasticity. 2 (2): 153–169. doi:10.3233/BPL-160043. ISSN 2213-6304. PMC 5928571. PMID 29765854.
- Haskell-Ramsay, Crystal F.; Schmitt, Jeroen; Actis-Goretta, Lucas (August 2018). "The Impact of Epicatechin on Human Cognition: The Role of Cerebral Blood Flow". Nutrients. 10 (8): 986. doi:10.3390/nu10080986. ISSN 2072-6643. PMC 6115745. PMID 30060538.
- Strasser, Barbara; Gostner, Johanna M.; Fuchs, Dietmar (January 2016). "Mood, food, and cognition: role of tryptophan and serotonin". Current Opinion in Clinical Nutrition and Metabolic Care. 19 (1): 55–61. doi:10.1097/MCO.0000000000000237. PMID 26560523. S2CID 12387611.
- Pranav, Joshi C. (2013). "A review on natural memory enhancers (nootropics)" (PDF). Unique Journal of Engineering and Advanced Sciences.
- Jongkees, Bryant J.; Hommel, Bernhard; Kühn, Simone; Colzato, Lorenza S. (1 November 2015). "Effect of tyrosine supplementation on clinical and healthy populations under stress or cognitive demands—A review". Journal of Psychiatric Research. 70: 50–57. doi:10.1016/j.jpsychires.2015.08.014. ISSN 0022-3956. PMID 26424423.
- McCarthy, Bozena; O'Neill, Graham; Abu-Ghannam, Nissreen (August 2022). "Potential Psychoactive Effects of Microalgal Bioactive Compounds for the Case of Sleep and Mood Regulation: Opportunities and Challenges". Marine Drugs. 20 (8): 493. doi:10.3390/md20080493. ISSN 1660-3397. PMC 9410000. PMID 36005495.
- Lee, Gihyun; Bae, Hyunsu (2017). "Therapeutic Effects of Phytochemicals and Medicinal Herbs on Depression". BioMed Research International. 2017: 1–11. doi:10.1155/2017/6596241. PMC 5414506. PMID 28503571.
- Venigalla, Madhuri; Gyengesi, Erika; Münch, Gerald (August 2015). "Curcumin and Apigenin – novel and promising therapeutics against chronic neuroinflammation in Alzheimer's disease". Neural Regeneration Research. 10 (8): 1181–1185. doi:10.4103/1673-5374.162686. ISSN 1673-5374. PMC 4590215. PMID 26487830.
- Salehi, Bahare; Venditti, Alessandro; Sharifi-Rad, Mehdi; Kręgiel, Dorota; Sharifi-Rad, Javad; Durazzo, Alessandra; Lucarini, Massimo; Santini, Antonello; Souto, Eliana B.; Novellino, Ettore; Antolak, Hubert; Azzini, Elena; Setzer, William N.; Martins, Natália (January 2019). "The Therapeutic Potential of Apigenin". International Journal of Molecular Sciences. 20 (6): 1305. doi:10.3390/ijms20061305. ISSN 1422-0067. PMC 6472148. PMID 30875872.
- Arcusa, Raúl; Villaño, Débora; Marhuenda, Javier; Cano, Miguel; Cerdà, Begoña; Zafrilla, Pilar (2022). "Potential Role of Ginger (Zingiber officinale Roscoe) in the Prevention of Neurodegenerative Diseases". Frontiers in Nutrition. 9: 809621. doi:10.3389/fnut.2022.809621. ISSN 2296-861X. PMC 8971783. PMID 35369082.
- Etheridge, Christopher John; Derbyshire, Emma (1 January 2020). "Herbal infusions and health: A review of findings from human studies, mechanisms and future research directions". Nutrition & Food Science. 50 (5): 969–985. doi:10.1108/NFS-08-2019-0263. ISSN 0034-6659. S2CID 211002387.
- Howes, Melanie-Jayne R.; Perry, Nicolette S.L.; Vásquez-Londoño, Carlos; Perry, Elaine K. (March 2020). "Role of phytochemicals as nutraceuticals for cognitive functions affected in ageing". British Journal of Pharmacology. 177 (6): 1294–1315. doi:10.1111/bph.14898. ISSN 0007-1188. PMC 7056459. PMID 31650528.
- Roe, Amy L.; Venkataraman, Arvind (September 2021). "The Safety and Efficacy of Botanicals with Nootropic Effects". Current Neuropharmacology. 19 (9): 1442–1467. doi:10.2174/1570159X19666210726150432. PMC 8762178. PMID 34315377.
- Hussain, S. M.; Syeda, A. F.; Alshammari, M.; Alnasser, S.; Alenzi, N. D.; Alanazi, S. T.; Nandakumar, K. (9 February 2022). "Cognition enhancing effect of rosemary (Rosmarinus officinalis L.) in lab animal studies: a systematic review and meta-analysis". Brazilian Journal of Medical and Biological Research. 55: e11593. doi:10.1590/1414-431X2021e11593. ISSN 0100-879X. PMC 8851910. PMID 35170682.
- Lewis, John E.; Poles, Jillian; Shaw, Delaney P.; Karhu, Elisa; Khan, Sher Ali; Lyons, Annabel E.; Sacco, Susana Barreiro; McDaniel, H. Reginald (26 August 2021). "The effects of twenty-one nutrients and phytonutrients on cognitive function: A narrative review". Journal of Clinical and Translational Research. 7 (4): 575–620. doi:10.18053/jctres.07.202104.014. ISSN 2424-810X. PMC 8445631. PMID 34541370.
- Suliman, Noor Azuin; Mat Taib, Che Norma; Mohd Moklas, Mohamad Aris; Adenan, Mohd Ilham; Hidayat Baharuldin, Mohamad Taufik; Basir, Rusliza (30 August 2016). "Establishing Natural Nootropics: Recent Molecular Enhancement Influenced by Natural Nootropic". Evidence-Based Complementary and Alternative Medicine. 2016: e4391375. doi:10.1155/2016/4391375. ISSN 1741-427X. PMC 5021479. PMID 27656235.
- Roschel, Hamilton; Gualano, Bruno; Ostojic, Sergej M.; Rawson, Eric S. (February 2021). "Creatine Supplementation and Brain Health". Nutrients. 13 (2): 586. doi:10.3390/nu13020586. ISSN 2072-6643. PMC 7916590. PMID 33578876.
- Dolan, Eimear; Gualano, Bruno; Rawson, Eric S. (2 January 2019). "Beyond muscle: the effects of creatine supplementation on brain creatine, cognitive processing, and traumatic brain injury". European Journal of Sport Science. 19 (1): 1–14. doi:10.1080/17461391.2018.1500644. ISSN 1746-1391. PMID 30086660. S2CID 51936612.
- Crawford, Cindy; Boyd, Courtney; Deuster, Patricia A. (1 November 2021). "Dietary Supplement Ingredients for Optimizing Cognitive Performance Among Healthy Adults: A Systematic Review". The Journal of Alternative and Complementary Medicine. 27 (11): 940–958. doi:10.1089/acm.2021.0135. ISSN 1075-5535. PMID 34370563. S2CID 236969310.
- Dyall, Simon C. (2015). "Long-chain omega-3 fatty acids and the brain: a review of the independent and shared effects of EPA, DPA and DHA". Frontiers in Aging Neuroscience. 7: 52. doi:10.3389/fnagi.2015.00052. ISSN 1663-4365. PMC 4404917. PMID 25954194.
- Singh, Jessica E. (1 September 2020). "Dietary Sources of Omega-3 Fatty Acids Versus Omega-3 Fatty Acid Supplementation Effects on Cognition and Inflammation". Current Nutrition Reports. 9 (3): 264–277. doi:10.1007/s13668-020-00329-x. ISSN 2161-3311. PMID 32621236. S2CID 220306807.
- Fadó, Rut; Molins, Anna; Rojas, Rocío; Casals, Núria (January 2022). "Feeding the Brain: Effect of Nutrients on Cognition, Synaptic Function, and AMPA Receptors". Nutrients. 14 (19): 4137. doi:10.3390/nu14194137. ISSN 2072-6643. PMC 9572450. PMID 36235789.
- Weiser, Michael J.; Butt, Christopher M.; Mohajeri, M. Hasan (February 2016). "Docosahexaenoic Acid and Cognition throughout the Lifespan". Nutrients. 8 (2): 99. doi:10.3390/nu8020099. ISSN 2072-6643. PMC 4772061. PMID 26901223.
- [163][164][149][165][166][97] and other nutrients and phytonutrients[158][162]
- Muscaritoli, Maurizio (2021). "The Impact of Nutrients on Mental Health and Well-Being: Insights From the Literature". Frontiers in Nutrition. 8: 656290. doi:10.3389/fnut.2021.656290. ISSN 2296-861X. PMC 7982519. PMID 33763446.
- Venkatramanan, Sudha; Armata, Ilianna E; Strupp, Barbara J; Finkelstein, Julia L (1 September 2016). "Vitamin B-12 and Cognition in Children". Advances in Nutrition. 7 (5): 879–888. doi:10.3945/an.115.012021. ISSN 2161-8313. PMC 5015033. PMID 27633104.
Despite the high prevalence of vitamin B-12 insufficiency and associated risk of adverse cognitive outcomes in children, to our knowledge, no studies to date have been conducted to examine the effects of vitamin B-12 supplementation on cognition in children.
- Benton, David (January 2013). "To establish the parameters of optimal nutrition do we need to consider psychological in addition to physiological parameters?". Molecular Nutrition & Food Research. 57 (1): 6–19. doi:10.1002/mnfr.201200477. PMID 23038656.
The decarboyxlase enzymes have pyridoxal phosphate as a coenzyme, the form in which vitamin B6 occurs most commonly in the diet. Yet there is evidence of marginal intakes of this vitamin: e.g. using a biochemical measure of pyridoxal phosphate status there was a subgroup of about 10% of British school children who were deficient [89]. In young British adults 27.7% of males and 36.6% of females were deficient as judged by the same measure
- Stevens, Gretchen A.; Beal, Ty; Mbuya, Mduduzi N. N.; Luo, Hanqi; Neufeld, Lynnette M.; Addo, O. Yaw; Adu-Afarwuah, Seth; Alayón, Silvia; Bhutta, Zulfiqar; Brown, Kenneth H.; Jefferds, Maria Elena; Engle-Stone, Reina; Fawzi, Wafaie; Hess, Sonja Y.; Johnston, Robert; Katz, Joanne; Krasevec, Julia; McDonald, Christine M.; Mei, Zuguo; Osendarp, Saskia; Paciorek, Christopher J.; Petry, Nicolai; Pfeiffer, Christine M.; Ramirez-Luzuriaga, Maria J.; Rogers, Lisa M.; Rohner, Fabian; Sethi, Vani; Suchdev, Parminder S.; Tessema, Masresha; Villapando, Salvador; Wieringa, Frank T.; Williams, Anne M.; Woldeyahannes, Meseret; Young, Melissa F. (1 November 2022). "Micronutrient deficiencies among preschool-aged children and women of reproductive age worldwide: a pooled analysis of individual-level data from population-representative surveys". The Lancet Global Health. 10 (11): e1590–e1599. doi:10.1016/S2214-109X(22)00367-9. ISSN 2214-109X. PMID 36240826. S2CID 252857990.
- Enderami, Athena; Zarghami, Mehran; Darvishi-Khezri, Hadi (1 October 2018). "The effects and potential mechanisms of folic acid on cognitive function: a comprehensive review". Neurological Sciences. 39 (10): 1667–1675. doi:10.1007/s10072-018-3473-4. ISSN 1590-3478. PMID 29936555. S2CID 49421574.
- Philippou, Elena; Constantinou, Marios (1 March 2014). "The Influence of Glycemic Index on Cognitive Functioning: A Systematic Review of the Evidence". Advances in Nutrition. 5 (2): 119–130. doi:10.3945/an.113.004960. ISSN 2161-8313. PMC 3951795. PMID 24618754.
- Rebelos, Eleni; Rinne, Juha O.; Nuutila, Pirjo; Ekblad, Laura L. (January 2021). "Brain Glucose Metabolism in Health, Obesity, and Cognitive Decline—Does Insulin Have Anything to Do with It? A Narrative Review". Journal of Clinical Medicine. 10 (7): 1532. doi:10.3390/jcm10071532. ISSN 2077-0383. PMC 8038699. PMID 33917464.
- Gubert, Carolina; Hannan, Anthony J. (November 2021). "Exercise mimetics: harnessing the therapeutic effects of physical activity". Nature Reviews Drug Discovery. 20 (11): 862–879. doi:10.1038/s41573-021-00217-1. ISSN 1474-1784. PMID 34103713. S2CID 235379365.
- Yamada, Yujiro; Frith, Emily M.; Wong, Vickie; Spitz, Robert W.; Bell, Zachary W.; Chatakondi, Raksha N.; Abe, Takashi; Loenneke, Jeremy P. (1 June 2021). "Acute exercise and cognition: A review with testable questions for future research into cognitive enhancement with blood flow restriction". Medical Hypotheses. 151: 110586. doi:10.1016/j.mehy.2021.110586. ISSN 0306-9877. PMID 33848917. S2CID 233233538.
- Taubert, Marco; Villringer, Arno; Lehmann, Nico (2015). "Endurance Exercise as an "Endogenous" Neuro-enhancement Strategy to Facilitate Motor Learning". Frontiers in Human Neuroscience. 9: 692. doi:10.3389/fnhum.2015.00692. ISSN 1662-5161. PMC 4714627. PMID 26834602.
- Asua, Diego; Bougamra, Ghassen; Calleja-Felipe, María; Morales, Miguel; Knafo, Shira (1 February 2018). "Peptides Acting as Cognitive Enhancers". Neuroscience. 370: 81–87. doi:10.1016/j.neuroscience.2017.10.002. ISSN 0306-4522. PMID 29030286. S2CID 10269993.
- Wright, John W.; Harding, Joseph W. (1 January 2015). "The Brain Hepatocyte Growth Factor/c-Met Receptor System: A New Target for the Treatment of Alzheimer's Disease". Journal of Alzheimer's Disease. 45 (4): 985–1000. doi:10.3233/JAD-142814. ISSN 1387-2877. PMID 25649658.
- Ho, Jean K.; Nation, Daniel A. (1 September 2018). "Cognitive benefits of angiotensin IV and angiotensin-(1–7): A systematic review of experimental studies". Neuroscience & Biobehavioral Reviews. 92: 209–225. doi:10.1016/j.neubiorev.2018.05.005. ISSN 0149-7634. PMC 8916541. PMID 29733881.
- Hallberg, Mathias; Larhed, Mats (2020). "From Angiotensin IV to Small Peptidemimetics Inhibiting Insulin-Regulated Aminopeptidase". Frontiers in Pharmacology. 11: 590855. doi:10.3389/fphar.2020.590855. ISSN 1663-9812. PMC 7593869. PMID 33178027.
- Stern, Sarah A.; Alberini, Cristina M. (January 2013). "Mechanisms of memory enhancement". WIREs Systems Biology and Medicine. 5 (1): 37–53. doi:10.1002/wsbm.1196. ISSN 1939-5094. PMC 3527655. PMID 23151999.
- Hofmann, Stefan G.; Fang, Angela; Gutner, Cassidy A. (1 January 2014). "Cognitive enhancers for the treatment of anxiety disorders". Restorative Neurology and Neuroscience. 32 (1): 183–195. doi:10.3233/RNN-139002. ISSN 0922-6028. PMID 23542909.
- Diekelmann, Susanne (2014). "Sleep for cognitive enhancement". Frontiers in Systems Neuroscience. 8: 46. doi:10.3389/fnsys.2014.00046. ISSN 1662-5137. PMC 3980112. PMID 24765066.
- Merlo, Emiliano; Milton, Amy L; Everitt, Barry J (1 August 2015). "Enhancing cognition by affecting memory reconsolidation" (PDF). Current Opinion in Behavioral Sciences. 4: 41–47. doi:10.1016/j.cobeha.2015.02.003. ISSN 2352-1546. S2CID 53170506.
- Desibhatla, Mukund (1 May 2021). "The Development and Evaluation of Novel DA Transport Inhibitors and their Effects on Effort-Related Motivation: A Review". Honors Scholar Theses.
- Froestl, Wolfgang; Muhs, Andreas; Pfeifer, Andrea (1 January 2012). "Cognitive Enhancers (Nootropics). Part 1: Drugs Interacting with Receptors". Journal of Alzheimer's Disease. 32 (4): 793–887. doi:10.3233/JAD-2012-121186. ISSN 1387-2877. PMID 22886028.
- Partin, Kathryn M (1 February 2015). "AMPA receptor potentiators: from drug design to cognitive enhancement". Current Opinion in Pharmacology. 20: 46–53. doi:10.1016/j.coph.2014.11.002. ISSN 1471-4892. PMC 4318786. PMID 25462292.
- Þorsteinsson, Haraldur; Karlsson, KarlÆgir (12 August 2009). "Is Sleep Beyond Our Control?". The Open Sleep Journal. 2 (1): 48–55. doi:10.2174/1874620900902010048.
- Kato, Taro; Duman, Ronald S. (1 January 2020). "Rapastinel, a novel glutamatergic agent with ketamine-like antidepressant actions: Convergent mechanisms". Pharmacology Biochemistry and Behavior. 188: 172827. doi:10.1016/j.pbb.2019.172827. ISSN 0091-3057. PMID 31733218. S2CID 207976034.
- Burgdorf, Jeffrey; Zhang, Xiao-lei; Weiss, Craig; Matthews, Elizabeth; Disterhoft, John F.; Stanton, Patric K.; Moskal, Joseph R. (1 April 2011). "The N-methyl-d-aspartate receptor modulator GLYX-13 enhances learning and memory, in young adult and learning impaired aging rats". Neurobiology of Aging. 32 (4): 698–706. doi:10.1016/j.neurobiolaging.2009.04.012. ISSN 0197-4580. PMC 3035742. PMID 19446371.
- R. Moskal, Joseph; S. Burgdorf, Jeffrey; K. Stanton, Patric; A. Kroes, Roger; F. Disterhoft, John; M. Burch, Ronald; Amin Khan, M. (2017). "The Development of Rapastinel (Formerly GLYX-13); A Rapid Acting and Long Lasting Antidepressant". Current Neuropharmacology. 15 (1): 47–56. doi:10.2174/1570159X14666160321122703. PMC 5327451. PMID 26997507.
- Costa-Mattioli, Mauro; Walter, Peter (24 April 2020). "The integrated stress response: From mechanism to disease". Science. 368 (6489): eaat5314. doi:10.1126/science.aat5314. ISSN 0036-8075. PMC 8997189. PMID 32327570.
- Jin, Yang; Saatcioglu, Fahri (1 February 2020). "Targeting the Unfolded Protein Response in Hormone-Regulated Cancers". Trends in Cancer. 6 (2): 160–171. doi:10.1016/j.trecan.2019.12.001. ISSN 2405-8033. PMID 32061305. S2CID 211136354.
- Tardner, P. (2020-08-30). "The use of citicoline for the treatment of cognitive decline and cognitive impairment: A meta-analysis of pharmacological literature • International Journal of Environmental Science & Technology". International Journal of Environmental Science & Technology. Retrieved 2020-08-31.
- Atack, John R. (2011). "GABAA Receptor Subtype-Selective Modulators. II. α5-Selective Inverse Agonists for Cognition Enhancement". Current Topics in Medicinal Chemistry. 11 (9): 1203–1214. doi:10.2174/156802611795371314. PMID 21050171.
- Kleczkowska, Patrycja (January 2022). "Chimeric Structures in Mental Illnesses—"Magic" Molecules Specified for Complex Disorders". International Journal of Molecular Sciences. 23 (7): 3739. doi:10.3390/ijms23073739. ISSN 1422-0067. PMC 8998808. PMID 35409098.
- Sharma, Horrick; Santra, Soumava; Dutta, Aloke (November 2015). "Triple reuptake inhibitors as potential next-generation antidepressants: a new hope?". Future Medicinal Chemistry. 7 (17): 2385–2406. doi:10.4155/fmc.15.134. PMC 4976848. PMID 26619226.
- Subbaiah, Murugaiah A. M. (22 March 2018). "Triple Reuptake Inhibitors as Potential Therapeutics for Depression and Other Disorders: Design Paradigm and Developmental Challenges". Journal of Medicinal Chemistry. 61 (6): 2133–2165. doi:10.1021/acs.jmedchem.6b01827. ISSN 0022-2623. PMID 28731336.
- Jacobson, Laura H.; Hoyer, Daniel; Lecea, Luis (May 2022). "Hypocretins (orexins): The ultimate translational neuropeptides". Journal of Internal Medicine. 291 (5): 533–556. doi:10.1111/joim.13406. ISSN 0954-6820. PMID 35043499. S2CID 248119793.
- Seigneur, Erica; de Lecea, Luis (1 December 2020). "Hypocretin (Orexin) Replacement Therapies". Medicine in Drug Discovery. 8: 100070. doi:10.1016/j.medidd.2020.100070. ISSN 2590-0986. S2CID 225129746.
- Alhusaini, Mera; Eissa, Nermin; Saad, Ali K.; Beiram, Rami; Sadek, Bassem (2022). "Revisiting Preclinical Observations of Several Histamine H3 Receptor Antagonists/Inverse Agonists in Cognitive Impairment, Anxiety, Depression, and Sleep–Wake Cycle Disorder". Frontiers in Pharmacology. 13: 861094. doi:10.3389/fphar.2022.861094. ISSN 1663-9812. PMC 9198498. PMID 35721194.
- Shinde, Anil; Subramanian, Ramkumar; Palacharla, Raghava; Benade, Vijay; Abraham, Renny; Kamuju, Venkatesh; Pandey, Santosh; Badange, Rajesh; Achanta, Pramod Kumar; Nirogi, Ramakrishna (1 May 2021). "004 Samelisant (SUVN-G3031), Differentiating features over current treatments of narcolepsy". Sleep. 44 (Supplement_2): A2. doi:10.1093/sleep/zsab072.003. ISSN 0161-8105.
Recent research has described a procognitive effect of samelisant, an inverse agonist of H3 receptors, in animal models of schizophrenia [157]. Nevertheless, more studies are required.
- Poulose, Shibu M.; Thangthaeng, Nopporn; Miller, Marshall G.; Shukitt-Hale, Barbara (1 October 2015). "Effects of pterostilbene and resveratrol on brain and behavior". Neurochemistry International. 89: 227–233. doi:10.1016/j.neuint.2015.07.017. ISSN 0197-0186. PMID 26212523. S2CID 33577543.
- McCormack, Denise; McFadden, David (4 April 2013). "A Review of Pterostilbene Antioxidant Activity and Disease Modification". Oxidative Medicine and Cellular Longevity. 2013: e575482. doi:10.1155/2013/575482. ISSN 1942-0900. PMC 3649683. PMID 23691264.
- Buntwal, Luke; Sassi, Martina; Morgan, Alwena H.; Andrews, Zane B.; Davies, Jeffrey S. (1 November 2019). "Ghrelin-Mediated Hippocampal Neurogenesis: Implications for Health and Disease". Trends in Endocrinology & Metabolism. 30 (11): 844–859. doi:10.1016/j.tem.2019.07.001. ISSN 1043-2760. PMID 31445747. S2CID 201126380.
- Morgan, A. H.; Andrews, Z. B.; Davies, J. S. (October 2017). "Less is more: Caloric regulation of neurogenesis and adult brain function". Journal of Neuroendocrinology. 29 (10): e12512. doi:10.1111/jne.12512. PMID 28771924. S2CID 3070497.
- Harwell, Victoria; Fasinu, Pius (1 September 2020). "Pitolisant and Other Histamine-3 Receptor Antagonists—An Update on Therapeutic Potentials and Clinical Prospects". Medicines. 7 (9): 55. doi:10.3390/medicines7090055. PMC 7554886. PMID 32882898.
- Schlicker, Eberhard; Kathmann, Markus (2017). "Role of the Histamine H3 Receptor in the Central Nervous System". Histamine and Histamine Receptors in Health and Disease. Springer International Publishing. pp. 277–299. ISBN 978-3-319-58194-1.
- Lapin, Izyaslav (7 June 2006). "Phenibut (β-Phenyl-GABA): A Tranquilizer and Nootropic Drug". CNS Drug Reviews. 7 (4): 471–481. doi:10.1111/j.1527-3458.2001.tb00211.x. PMC 6494145. PMID 11830761.
- Kupats, Einars; Vrublevska, Jelena; Zvejniece, Baiba; Vavers, Edijs; Stelfa, Gundega; Zvejniece, Liga; Dambrova, Maija (September 2020). "Safety and Tolerability of the Anxiolytic and Nootropic Drug Phenibut: A Systematic Review of Clinical Trials and Case Reports". Pharmacopsychiatry. 53 (5): 201–208. doi:10.1055/a-1151-5017. ISSN 0176-3679. PMID 32340063. S2CID 216593771.
- Jędrejko, Karol; Lazur, Jan; Muszyńska, Bożena (February 2021). "Risk Associated with the Use of Selected Ingredients in Food Supplements". Chemistry & Biodiversity. 18 (2): e2000686. doi:10.1002/cbdv.202000686. ISSN 1612-1872. PMID 33410585. S2CID 230821273.
- Batalla, Albert; Bos, Julian; Postma, Amber; Bossong, Matthijs G. (2021). "The Impact of Cannabidiol on Human Brain Function: A Systematic Review". Frontiers in Pharmacology. 11: 618184. doi:10.3389/fphar.2020.618184. ISSN 1663-9812. PMC 7858248. PMID 33551817.
- Colizzi, Marco; Ruggeri, Mirella; Bhattacharyya, Sagnik (2020). "Unraveling the Intoxicating and Therapeutic Effects of Cannabis Ingredients on Psychosis and Cognition". Frontiers in Psychology. 11: 833. doi:10.3389/fpsyg.2020.00833. ISSN 1664-1078. PMC 7247841. PMID 32528345.
- Daubner, Johanna; Arshaad, Muhammad Imran; Henseler, Christina; Hescheler, Jürgen; Ehninger, Dan; Broich, Karl; Rawashdeh, Oliver; Papazoglou, Anna; Weiergräber, Marco (13 January 2021). "Pharmacological Neuroenhancement: Current Aspects of Categorization, Epidemiology, Pharmacology, Drug Development, Ethics, and Future Perspectives". Neural Plasticity. 2021: e8823383. doi:10.1155/2021/8823383. ISSN 2090-5904. PMC 7817276. PMID 33519929.
- Zajdel, P; Bednarski, M; Sapa, J; Nowak, G (2015). "Ergotamine and nicergoline – facts and myths". Pharmacol Rep. 67 (2): 360–363. doi:10.1016/j.pharep.2014.10.010. PMID 25712664. S2CID 22768662.
- Gerbarg, Patricia L.; Brown, Richard P. (March 2013). "Phytomedicines for Prevention and Treatment of Mental Health Disorders". Psychiatric Clinics of North America. 36 (1): 37–47. doi:10.1016/j.psc.2012.12.004. PMID 23538075.
- Clayton, Paul; Hill, Mariko; Bogoda, Nathasha; Subah, Silma; Venkatesh, Ruchitha (January 2021). "Palmitoylethanolamide: A Natural Compound for Health Management". International Journal of Molecular Sciences. 22 (10): 5305. doi:10.3390/ijms22105305. ISSN 1422-0067. PMC 8157570. PMID 34069940.
- Singh, Alok; Purohit, Vinay (1 June 2019). "A critical review of pyritinol". Drugs & Therapy Perspectives. 35 (6): 278–282. doi:10.1007/s40267-019-00623-x. ISSN 1179-1977. S2CID 256373631.
- Zimerman, Maximo; Nitsch, M; Giraux, P; Gerloff, C; Cohen, LG; Hummel, FC (2013). "Neuroenhancement of the Aging Brain: Restoring Skill Acquisition in Old Subjects". Annals of Neurology. 73 (1): 10–15. doi:10.1002/ana.23761. PMC 4880032. PMID 23225625.
- Meinzer, Marcus; Antonenko, D; Lindenberg, R; Hetzer, S; Ulm, L; Avirame, K; Flaisch, T; Flöel, A (2012). "Electrical brain stimulation improves cognitive performance by modulating functional connectivity and task-specific activation". The Journal of Neuroscience. 32 (5): 1859–1866. doi:10.1523/JNEUROSCI.4812-11.2012. PMC 6703352. PMID 22302824.
- Grover, Shrey; Wen, Wen; Viswanathan, Vighnesh; Gill, Christopher T.; Reinhart, Robert M. G. (September 2022). "Long-lasting, dissociable improvements in working memory and long-term memory in older adults with repetitive neuromodulation". Nature Neuroscience. 25 (9): 1237–1246. doi:10.1038/s41593-022-01132-3. ISSN 1546-1726. PMC 10068908. PMID 35995877. S2CID 251742309.
- News article about the study: LaMotte, Sandee. "Brain stimulation improves short-term memory in older adults, study finds". CNN. Retrieved 15 September 2022.
- Kupsch, Andreas; Benecke, Reiner; Müller, Jörg; Trottenberg, Thomas; Schneider, Gerd-Helge; Poewe, Werner; Eisner, Wilhelm; Wolters, Alexander; Müller, Jan-Uwe; Deuschl, Günther; Pinsker, Marcus O.; Skogseid, Inger Marie; Roeste, Geir Ketil; Vollmer-Haase, Juliane; Brentrup, Angela; Krause, Martin; Tronnier, Volker; Schnitzler, Alfons; Voges, Jürgen; Nikkhah, Guido; Vesper, Jan; Naumann, Markus; Volkmann, Jens; Deep-Brain Stimulation for Dystonia Study Group (2006). "Pallidal Deep-Brain Stimulation in Primary Generalized or Segmental Dystonia". New England Journal of Medicine. 355 (19): 1978–1990. doi:10.1056/NEJMoa063618. PMID 17093249.
- Notbohm, Annika; Kurths, Jürgen; Herrmann, Christoph S. (2016). "Modification of Brain Oscillations via Rhythmic Light Stimulation Provides Evidence for Entrainment but Not for Superposition of Event-Related Responses". Frontiers in Human Neuroscience. 10: 10. doi:10.3389/fnhum.2016.00010. ISSN 1662-5161. PMC 4737907. PMID 26869898.
- Ding, Nai; Simon, Jonathan Z. (2014). "Cortical entrainment to continuous speech: functional roles and interpretations". Frontiers in Human Neuroscience. 8: 311. doi:10.3389/fnhum.2014.00311. ISSN 1662-5161. PMC 4036061. PMID 24904354.
- Thaut, Michael H. (2015-01-01), Altenmüller, Eckart; Finger, Stanley; Boller, François (eds.), "Chapter 13 - The discovery of human auditory–motor entrainment and its role in the development of neurologic music therapy", Progress in Brain Research, Music, Neurology, and Neuroscience: Evolution, the Musical Brain, Medical Conditions, and Therapies, Elsevier, 217: 253–266, doi:10.1016/bs.pbr.2014.11.030, ISBN 9780444635518, PMID 25725919, retrieved 2021-12-01
- Cantor, David S.; Evans, James R. (2013-10-18). Clinical Neurotherapy: Application of Techniques for Treatment. Academic Press. ISBN 9780123972910.
- Diep, Charmaine; Ftouni, Suzanne; Manousakis, Jessica E; Nicholas, Christian L; Drummond, Sean P A; Anderson, Clare (2019-11-06). "Acoustic slow wave sleep enhancement via a novel, automated device improves executive function in middle-aged men". Sleep. 43 (1). doi:10.1093/sleep/zsz197. ISSN 0161-8105. PMID 31691831.
- Michael, Elizabeth; Covarrubias, Lorena Santamaria; Leong, Victoria; Kourtzi, Zoe (9 November 2022). "Learning at your brain's rhythm: individualized entrainment boosts learning for perceptual decisions". Cerebral Cortex. 33 (9): 5382–5394. doi:10.1093/cercor/bhac426. PMC 10152088. PMID 36352510.
- News article about the study: "Brain-frequency primer accelerates learning and retention". New Atlas. 1 February 2023. Archived from the original on 15 February 2023. Retrieved 15 February 2023.
- Lucke, Jayne; Partridge, Brad (1 August 2013). "Towards a Smart Population: A Public Health Framework for Cognitive Enhancement". Neuroethics. 6 (2): 419–427. doi:10.1007/s12152-012-9167-3. ISSN 1874-5504. S2CID 255510188.
- O'Connor, Cliodhna; Nagel, Saskia K. (2017). "Neuro-Enhancement Practices across the Lifecourse: Exploring the Roles of Relationality and Individualism". Frontiers in Sociology. 2. doi:10.3389/fsoc.2017.00001. ISSN 2297-7775.
- Cancer, Alice; Schulz, Peter J.; Castaldi, Silvana; Antonietti, Alessandro (1 December 2018). "Neuroethical Issues in Cognitive Enhancement: the Undergraduates' Point of View". Journal of Cognitive Enhancement. 2 (4): 323–330. doi:10.1007/s41465-018-0110-3. ISSN 2509-3304. S2CID 256624023.
- Heller, Sebastian; Tibubos, Ana Nanette; Hoff, Thilo A.; Werner, Antonia M.; Reichel, Jennifer L.; Mülder, Lina M.; Schäfer, Markus; Pfirrmann, Daniel; Stark, Birgit; Rigotti, Thomas; Simon, Perikles; Beutel, Manfred E.; Letzel, Stephan; Dietz, Pavel (18 January 2022). "Potential risk groups and psychological, psychosocial, and health behavioral predictors of pharmacological neuroenhancement among university students in Germany". Scientific Reports. 12 (1): 937. Bibcode:2022NatSR..12..937H. doi:10.1038/s41598-022-04891-y. ISSN 2045-2322. PMC 8766436. PMID 35042938.
- Julvez, Jordi; López-Vicente, Mónica; Warembourg, Charline; Maitre, Lea; Philippat, Claire; Gützkow, Kristine B.; Guxens, Monica; Evandt, Jorunn; Andrusaityte, Sandra; Burgaleta, Miguel; Casas, Maribel; Chatzi, Leda; de Castro, Montserrat; Donaire-González, David; Gražulevičienė, Regina; Hernandez-Ferrer, Carles; Heude, Barbara; Mceachan, Rosie; Mon-Williams, Mark; Nieuwenhuijsen, Mark; Robinson, Oliver; Sakhi, Amrit K.; Sebastian-Galles, Nuria; Slama, Remy; Sunyer, Jordi; Tamayo-Uria, Ibon; Thomsen, Cathrine; Urquiza, Jose; Vafeiadi, Marina; Wright, John; Basagaña, Xavier; Vrijheid, Martine (1 September 2021). "Early life multiple exposures and child cognitive function: A multi-centric birth cohort study in six European countries". Environmental Pollution. 284: 117404. doi:10.1016/j.envpol.2021.117404. ISSN 0269-7491. PMC 8287594. PMID 34077897.
- Costa, Lucio G.; Cole, Toby B.; Dao, Khoi; Chang, Yu-Chi; Coburn, Jacki; Garrick, Jacqueline M. (June 2020). "Effects of air pollution on the nervous system and its possible role in neurodevelopmental and neurodegenerative disorders". Pharmacology & Therapeutics. 210: 107523. doi:10.1016/j.pharmthera.2020.107523. ISSN 1879-016X. PMC 7245732. PMID 32165138.
- Volk, Heather E.; Perera, Frederica; Braun, Joseph M.; Kingsley, Samantha L.; Gray, Kimberly; Buckley, Jessie; Clougherty, Jane E.; Croen, Lisa A.; Eskenazi, Brenda; Herting, Megan; Just, Allan C.; Kloog, Itai; Margolis, Amy; McClure, Leslie A.; Miller, Rachel; Levine, Sarah; Wright, Rosalind (1 May 2021). "Prenatal air pollution exposure and neurodevelopment: A review and blueprint for a harmonized approach within ECHO". Environmental Research. 196: 110320. Bibcode:2021ER....196k0320V. doi:10.1016/j.envres.2020.110320. ISSN 0013-9351. PMC 8060371. PMID 33098817.
- Shang, Li; Yang, Liren; Yang, Wenfang; Huang, Liyan; Qi, Cuifang; Yang, Zixuan; Fu, Zhuxuan; Chung, Mei Chun (1 July 2020). "Effects of prenatal exposure to NO2 on children's neurodevelopment: a systematic review and meta-analysis". Environmental Science and Pollution Research. 27 (20): 24786–24798. doi:10.1007/s11356-020-08832-y. ISSN 1614-7499. PMC 7329770. PMID 32356052. S2CID 216650267.
- Cedeño Laurent, Jose Guillermo; MacNaughton, Piers; Jones, Emily; Young, Anna S; Bliss, Maya; Flanigan, Skye; Vallarino, Jose; Chen, Ling Jyh; Cao, Xiaodong; Allen, Joseph G (1 September 2021). "Associations between acute exposures to PM2.5 and carbon dioxide indoors and cognitive function in office workers: a multicountry longitudinal prospective observational study". Environmental Research Letters. 16 (9): 094047. Bibcode:2021ERL....16i4047C. doi:10.1088/1748-9326/ac1bd8. ISSN 1748-9326. PMC 8942432. PMID 35330988. S2CID 237462480.
- McFarland, Michael J.; Hauer, Matt E.; Reuben, Aaron (15 March 2022). "Half of US population exposed to adverse lead levels in early childhood". Proceedings of the National Academy of Sciences. 119 (11): e2118631119. Bibcode:2022PNAS..11918631M. doi:10.1073/pnas.2118631119. ISSN 0027-8424. PMC 8931364. PMID 35254913.
- University press release: "Lead exposure in last century shrunk IQ scores of half of Americans". Duke University. Retrieved 18 April 2022.
- Dündar-Coecke, Selma (1 December 2021). "Future avenues for education and neuroenhancement". New Ideas in Psychology. 63: 100875. doi:10.1016/j.newideapsych.2021.100875. ISSN 0732-118X. S2CID 236312270.
- Voinea, Cristina; Vică, Constantin; Mihailov, Emilian; Savulescu, Julian (1 August 2020). "The Internet as Cognitive Enhancement". Science and Engineering Ethics. 26 (4): 2345–2362. doi:10.1007/s11948-020-00210-8. ISSN 1471-5546. PMC 7417391. PMID 32253711.
- Naeem, Noor-i-Kiran; Yusoff, Muhamad Saiful Bahri; Hadie, Siti Nurma Hanim; Ismail, Irwan Mahazir; Iqbal, Haris (28 February 2023). "Understanding the Functional Components of Technology-Enhanced Learning Environment in Medical Education: A Scoping Review". Medical Science Educator. 33 (2): 595–609. doi:10.1007/s40670-023-01747-6. ISSN 2156-8650. PMC 9972326. PMID 37251205. S2CID 257254561.
- Drigas, Athanasios; Karyotaki, Maria (12 June 2014). "Learning Tools and Applications for Cognitive Improvement". International Journal of Engineering Pedagogy (IJEP). 4 (3): 71. doi:10.3991/ijep.v4i3.3665.
- Ortiz-Ospina, Esteban; Giattino, Charlie; Roser, Max (29 November 2020). "Time Use". Our World in Data. Retrieved 11 March 2023.
- Vedechkina, Maria; Borgonovi, Francesca (2021). "A Review of Evidence on the Role of Digital Technology in Shaping Attention and Cognitive Control in Children". Frontiers in Psychology. 12: 611155. doi:10.3389/fpsyg.2021.611155. ISSN 1664-1078. PMC 7943608. PMID 33716873.
- Hutchinson, Amanda D.; Wilson, Carlene (6 July 2011). "Improving nutrition and physical activity in the workplace: a meta-analysis of intervention studies". Health Promotion International. 27 (2): 238–249. doi:10.1093/heapro/dar035. ISSN 1460-2245. PMID 21733915.
- Gashaj, Venera; Dapp, Laura C.; Trninic, Dragan; Roebers, Claudia M. (1 December 2021). "The effect of video games, exergames and board games on executive functions in kindergarten and 2nd grade: An explorative longitudinal study". Trends in Neuroscience and Education. 25: 100162. doi:10.1016/j.tine.2021.100162. ISSN 2211-9493. PMID 34844694. S2CID 237631258.
- Caton, Amy; Bradshaw-Ward, Danita; Kinshuk, Kinshuk; Savenye, Wilhelmina (21 November 2022). "Future Directions for Digital Literacy Fluency using Cognitive Flexibility Research: A Review of Selected Digital Literacy Paradigms and Theoretical Frameworks". Journal of Learning for Development. 9 (3): 381–393. doi:10.56059/jl4d.v9i3.818. ISSN 2311-1550. S2CID 254004509.
- Wang, Feng; Kinzie, Mable B.; McGuire, Patrick; Pan, Edward (1 March 2010). "Applying Technology to Inquiry-Based Learning in Early Childhood Education". Early Childhood Education Journal. 37 (5): 381–389. doi:10.1007/s10643-009-0364-6. ISSN 1573-1707. S2CID 143666182.
- García-Pérez, Daniel; de Aldama, Carlos; Aguirre-Camacho, Aldo; González-Cuevas, Gustavo (March 2021). "Critical Thinking Assessment of Internet Inquiry and Argumentation in Text Generation". INTED2021 Proceedings. Vol. 1. pp. 5341–5347. doi:10.21125/inted.2021.1091. ISBN 978-84-09-27666-0. S2CID 233801659.
- Riegel, Ulrich; Mendl, Hans (4 May 2014). "What should religious education in Germany be about and how does religiosity fit into this picture? An empirical study of pre-service religious education teachers' beliefs on the aims of RE". Journal of Beliefs & Values. 35 (2): 165–174. doi:10.1080/13617672.2014.953300. ISSN 1361-7672. S2CID 144001680.
- Kittelmann Flensner, Karin (2017). Discourses of religion and secularism in religious education classrooms. Cham, Switzerland: Springer. ISBN 978-3-319-60949-2.
- Setyowidodo, I; Jatmiko, B; Susantini, E; Handayani, A D; Pramesti, Y S (1 April 2020). "The role of science project based peer interaction on improving collaborative skills and physical problem solving: a mini review". Journal of Physics: Conference Series. 1521 (2): 022032. Bibcode:2020JPhCS1521b2032S. doi:10.1088/1742-6596/1521/2/022032. S2CID 219448364.
- Norman, Geoff (1 January 2009). "Teaching basic science to optimize transfer". Medical Teacher. 31 (9): 807–811. doi:10.1080/01421590903049814. ISSN 0142-159X. PMID 19811185. S2CID 26691454.
- Kalyuga, Slava; Renkl, Alexander; Paas, Fred (1 June 2010). "Facilitating Flexible Problem Solving: A Cognitive Load Perspective". Educational Psychology Review. 22 (2): 175–186. doi:10.1007/s10648-010-9132-9. ISSN 1573-336X. S2CID 56420974.
- Edgren, Nora; Dubljević, Veljko (1 February 2023). "The ubiquuity of the fallacy of composition in cognitive enhancement and in education". Theoretical Medicine and Bioethics. 44 (1): 41–56. doi:10.1007/s11017-022-09595-y. ISSN 1573-1200. PMID 36273366. S2CID 253081249.
- Schneider, Felicitas; Horowitz, Alan; Lesch, Klaus-Peter; Dandekar, Thomas (21 January 2020). "Delaying memory decline: different options and emerging solutions". Translational Psychiatry. 10 (1): 13. doi:10.1038/s41398-020-0697-x. ISSN 2158-3188. PMC 7026464. PMID 32066684.
- Moreno, Jonathan; Gross, Michael L.; Becker, Jack; Hereth, Blake; Shortland, Neil D.; Evans, Nicholas G. (2022). "The ethics of AI-assisted warfighter enhancement research and experimentation: Historical perspectives and ethical challenges". Frontiers in Big Data. 5: 978734. doi:10.3389/fdata.2022.978734. ISSN 2624-909X. PMC 9500287. PMID 36156934.
- Smart, Paul (1 September 2017). "Extended Cognition and the Internet". Philosophy & Technology. 30 (3): 357–390. doi:10.1007/s13347-016-0250-2. ISSN 2210-5441. PMC 6961510. PMID 32010552.
- Smart, Paul R. (22 November 2013). "The Web-Extended Mind". Philosophical Engineering. John Wiley & Sons, Ltd: 116–133. doi:10.1002/9781118700143.ch8. ISBN 978-1-118-70014-3.
- Smart, Paul R. (2018). "Human-extended machine cognition" (PDF). Cognitive Systems Research. 49: 9–23. doi:10.1016/j.cogsys.2017.11.001. S2CID 4327856.
- Roh, Hyun Woong; Ryu, Hankyel; Jeong, Sooin; Han, Jieun; Park, Bumhee; Moon, So Young; Choi, Seong Hey; Son, Sang Joon; Hong, Chang Hyung (30 June 2022). "The effectiveness of a motivational enhancement smartphone application promoting lifestyle improvement for brain health: A randomized controlled trial". PLOS ONE. 17 (6): e0267806. Bibcode:2022PLoSO..1767806R. doi:10.1371/journal.pone.0267806. ISSN 1932-6203. PMC 9246229. PMID 35771740.
- "Cognitive collaboration". Deloitte Insights. Retrieved 17 March 2023.
- Nagao, Katashi (2019). "Symbiosis between Humans and Artificial Intelligence". Artificial Intelligence Accelerates Human Learning: Discussion Data Analytics. Springer. pp. 135–151. doi:10.1007/978-981-13-6175-3_6. ISBN 978-981-13-6175-3. S2CID 86713176.
- Smart, Paul R. "The Cognitive Machine: Cognitive Extension and Coalition Operations". Retrieved 17 March 2023.
- Hilbert, Martin (30 June 2020). "Digital technology and social change: the digital transformation of society from a historical perspective". Dialogues in Clinical Neuroscience. 22 (2): 189–194. doi:10.31887/DCNS.2020.22.2/mhilbert. PMC 7366943. PMID 32699519.
- "Future Today Institute – 2022 Tech Trends Report" (PDF). Retrieved 19 March 2023.
- Savulescu, Julian; Maslen, Hannah (2015). "Moral Enhancement and Artificial Intelligence: Moral AI?". Beyond Artificial Intelligence: The Disappearing Human-Machine Divide. Springer International Publishing. pp. 79–95. ISBN 978-3-319-09668-1.
- Aldama, C. (2020). "Cognitive enhancement or cognitive diminishing? digital technologies and challenges for education from a situated perspective" (PDF).
- de Aldama, Carlos; García-Pérez, Daniel (2022). "Social Challenges and Actions for Thinking and Reasoning in the Digital Age". The Palgrave Handbook of Global Social Change. Springer International Publishing. pp. 1–21. ISBN 978-3-030-87624-1.
- Benton, David (February 2012). "Vitamins and neural and cognitive developmental outcomes in children". Proceedings of the Nutrition Society. 71 (1): 14–26. doi:10.1017/S0029665111003247. ISSN 1475-2719. PMID 22030620. S2CID 5035428.
- "Scientists say active early learning shapes the adult brain". medicalxpress.com. Retrieved 14 June 2021.
- Farah, Martha J.; Sternberg, Saul; Nichols, Thomas A.; Duda, Jeffrey T.; Lohrenz, Terry; Luo, Yi; Sonnier, Libbie; Ramey, Sharon L.; Montague, Read; Ramey, Craig T. (2021-05-01). "Randomized Manipulation of Early Cognitive Experience Impacts Adult Brain Structure". Journal of Cognitive Neuroscience. 33 (6): 1197–1209. doi:10.1162/jocn_a_01709. hdl:10919/103551. PMID 34428792. S2CID 233638156.
- Yi, Chenju; Wang, Qi; Qu, Yibo; Niu, Jianqin; Oliver, Brian G.; Chen, Hui (June 2022). "In-utero exposure to air pollution and early-life neural development and cognition". Ecotoxicology and Environmental Safety. 238: 113589. doi:10.1016/j.ecoenv.2022.113589. PMID 35525116. S2CID 248561205.
- Zhou, Sherry; Rosenthal, David G.; Sherman, Scott; Zelikoff, Judith; Gordon, Terry; Weitzman, Michael (September 2014). "Physical, Behavioral, and Cognitive Effects of Prenatal Tobacco and Postnatal Secondhand Smoke Exposure". Current Problems in Pediatric and Adolescent Health Care. 44 (8): 219–241. doi:10.1016/j.cppeds.2014.03.007. PMC 6876620. PMID 25106748.
- Clifford, Angela; Lang, Linda; Chen, Ruoling; Anstey, Kaarin J.; Seaton, Anthony (1 May 2016). "Exposure to air pollution and cognitive functioning across the life course – A systematic literature review". Environmental Research. 147: 383–398. Bibcode:2016ER....147..383C. doi:10.1016/j.envres.2016.01.018. ISSN 0013-9351. PMID 26945620.
- Jurewicz, Joanna; Polańska, Kinga; Hanke, Wojciech (1 January 2013). "Exposure to widespread environmental toxicants and children's cognitive development and behavioral problems". International Journal of Occupational Medicine and Environmental Health. 26 (2): 185–204. doi:10.2478/s13382-013-0099-x. PMID 23715930. S2CID 37752328.
- [274][239][237][234][275][236][276][277]
- Poynton, Timothy A. (1 November 2005). "Computer literacy across the lifespan: a review with implications for educators". Computers in Human Behavior. 21 (6): 861–872. doi:10.1016/j.chb.2004.03.004. ISSN 0747-5632.
- Healey, Aleeya; Mendelsohn, Alan; Council on Early Childhood (2019). "Selecting Appropriate Toys for Young Children in the Digital Era". Pediatrics. 143 (1): e20183348. doi:10.1542/peds.2018-3348. PMID 30509931. S2CID 54553416.
- Schweikardt, Eric; Gross, Mark D (March 2007). "A Brief Survey of Distributed Computational Toys". 2007 First IEEE International Workshop on Digital Game and Intelligent Toy Enhanced Learning (DIGITEL'07). pp. 57–64. doi:10.1109/DIGITEL.2007.4. ISBN 978-0-7695-2801-4. S2CID 11106981.
- Merrick, Kathryn E. (2013). "Novelty and Beyond: Towards Combined Motivation Models and Integrated Learning Architectures". Intrinsically Motivated Learning in Natural and Artificial Systems. Springer. pp. 209–233. ISBN 978-3-642-32375-1.
- Demarin, Vida; Bedeković, Marina Roje; Puretić, Marijana Bosnar; Pašić, Marija Bošnjak (December 2016). "Arts, Brain and Cognition". Psychiatria Danubina. 28 (4): 343–348. ISSN 0353-5053. PMID 27855424.
- Dingle, Genevieve A.; Sharman, Leah S.; Bauer, Zoe; Beckman, Emma; Broughton, Mary; Bunzli, Emma; Davidson, Robert; Draper, Grace; Fairley, Sheranne; Farrell, Callyn; Flynn, Libby Maree; Gomersall, Sjaan; Hong, Mengxun; Larwood, Joel; Lee, Chiying; Lee, Jennifer; Nitschinsk, Lewis; Peluso, Natalie; Reedman, Sarah Elizabeth; Vidas, Dianna; Walter, Zoe C.; Wright, Olivia Renee Louise (2021). "How Do Music Activities Affect Health and Well-Being? A Scoping Review of Studies Examining Psychosocial Mechanisms". Frontiers in Psychology. 12: 713818. doi:10.3389/fpsyg.2021.713818. ISSN 1664-1078. PMC 8455907. PMID 34566791.
- Miller, Marshall G.; Shukitt-Hale, Barbara (13 June 2012). "Berry Fruit Enhances Beneficial Signaling in the Brain". Journal of Agricultural and Food Chemistry. 60 (23): 5709–5715. doi:10.1021/jf2036033. ISSN 0021-8561. PMID 22264107.
- Pribis, Peter; Shukitt-Hale, Barbara (July 2014). "Cognition: the new frontier for nuts and berries". The American Journal of Clinical Nutrition. 100: S347–S352. doi:10.3945/ajcn.113.071506. PMID 24871475.
- Clifford, Tom; Howatson, Glyn; West, Daniel J.; Stevenson, Emma J. (April 2015). "The Potential Benefits of Red Beetroot Supplementation in Health and Disease". Nutrients. 7 (4): 2801–2822. doi:10.3390/nu7042801. ISSN 2072-6643. PMC 4425174. PMID 25875121.
- George, Elena S; Daly, Robin M; Tey, Siew Ling; Brown, Rachel; Wong, Tommy Hon Ting; Tan, Sze-Yen (1 July 2022). "Perspective: Is it Time to Expand Research on "Nuts" to Include "Seeds"? Justifications and Key Considerations". Advances in Nutrition. 13 (4): 1016–1027. doi:10.1093/advances/nmac028. ISSN 2161-8313. PMC 9340969. PMID 35333288.
- Allen, Andrew P.; Dinan, Timothy G.; Clarke, Gerard; Cryan, John F. (April 2017). "A psychology of the human brain-gut-microbiome axis". Social and Personality Psychology Compass. 11 (4): e12309. doi:10.1111/spc3.12309. PMC 5530613. PMID 28804508.
- Basso, Melissa; Johnstone, Nicola; Knytl, Paul; Nauta, Arjen; Groeneveld, Andre; Cohen Kadosh, Kathrin (January 2022). "A Systematic Review of Psychobiotic Interventions in Children and Adolescents to Enhance Cognitive Functioning and Emotional Behavior". Nutrients. 14 (3): 614. doi:10.3390/nu14030614. ISSN 2072-6643. PMC 8840038. PMID 35276975.
- Sarkar, Amar; Harty, Siobhán; Lehto, Soili M.; Moeller, Andrew H.; Dinan, Timothy G.; Dunbar, Robin I. M.; Cryan, John F.; Burnet, Philip W. J. (1 July 2018). "The Microbiome in Psychology and Cognitive Neuroscience". Trends in Cognitive Sciences. 22 (7): 611–636. doi:10.1016/j.tics.2018.04.006. ISSN 1364-6613. PMID 29907531. S2CID 49223741.
- Vera-Santander, Victor E.; Hernández-Figueroa, Ricardo H.; Jiménez-Munguía, María T.; Mani-López, Emma; López-Malo, Aurelio (January 2023). "Health Benefits of Consuming Foods with Bacterial Probiotics, Postbiotics, and Their Metabolites: A Review". Molecules. 28 (3): 1230. doi:10.3390/molecules28031230. ISSN 1420-3049. PMC 9920731. PMID 36770898.
- Foshati, Sahar; Akhlaghi, Masoumeh; Babajafari, Siavash (30 August 2022). "The effect of pro-/synbiotic supplementation on the brain-derived neurotrophic factor: a systematic review and meta-analysis of randomized controlled trials". Food & Function. 13 (17): 8754–8765. doi:10.1039/D2FO01330D. ISSN 2042-650X. PMID 35943321. S2CID 251119998.
- [257][289][290][291][292][293]
- Gill, Louis-Nascan; Renault, Robin; Campbell, Emma; Rainville, Pierre; Khoury, Bassam (1 September 2020). "Mindfulness induction and cognition: A systematic review and meta-analysis". Consciousness and Cognition. 84: 102991. doi:10.1016/j.concog.2020.102991. ISSN 1053-8100. PMID 32739799. S2CID 220873007.
- Balban, Melis Yilmaz; Neri, Eric; Kogon, Manuela M.; Weed, Lara; Nouriani, Bita; Jo, Booil; Holl, Gary; Zeitzer, Jamie M.; Spiegel, David; Huberman, Andrew D. (17 January 2023). "Brief structured respiration practices enhance mood and reduce physiological arousal". Cell Reports Medicine. 4 (1): 100895. doi:10.1016/j.xcrm.2022.100895. ISSN 2666-3791. PMC 9873947. PMID 36630953.
- News article about the study: Gulzar, Ayesha (13 February 2023). "This 5-minute breathing technique can reduce anxiety and stress". interestingengineering.com. Archived from the original on 15 February 2023. Retrieved 16 February 2023.
- Parashar, Arun; Udayabanu, Malairaman (1 January 2016). "Gut microbiota regulates key modulators of social behavior". European Neuropsychopharmacology. 26 (1): 78–91. doi:10.1016/j.euroneuro.2015.11.002. ISSN 0924-977X. PMID 26613639. S2CID 39691319.
- Gudden, Jip; Arias Vasquez, Alejandro; Bloemendaal, Mirjam (September 2021). "The Effects of Intermittent Fasting on Brain and Cognitive Function". Nutrients. 13 (9): 3166. doi:10.3390/nu13093166. ISSN 2072-6643. PMC 8470960. PMID 34579042.
- Troynikov, Olga; Watson, Christopher G.; Nawaz, Nazia (1 December 2018). "Sleep environments and sleep physiology: A review". Journal of Thermal Biology. 78: 192–203. doi:10.1016/j.jtherbio.2018.09.012. ISSN 0306-4565. PMID 30509635. S2CID 54565447.
- Lacaux, Célia; Andrillon, Thomas; Bastoul, Céleste; Idir, Yannis; Fonteix-Galet, Alexandrine; Arnulf, Isabelle; Oudiette, Delphine (10 December 2021). "Sleep onset is a creative sweet spot". Science Advances. 7 (50): eabj5866. Bibcode:2021SciA....7.5866L. doi:10.1126/sciadv.abj5866. ISSN 2375-2548. PMC 8654287. PMID 34878849.
- Lewis, Penelope A.; Knoblich, Günther; Poe, Gina (1 June 2018). "How Memory Replay in Sleep Boosts Creative Problem-Solving". Trends in Cognitive Sciences. 22 (6): 491–503. doi:10.1016/j.tics.2018.03.009. ISSN 1364-6613. PMC 7543772. PMID 29776467.
- Walker, Matthew P. (2009). "The Role of Sleep in Cognition and Emotion". Annals of the New York Academy of Sciences. 1156 (1): 168–197. Bibcode:2009NYASA1156..168W. doi:10.1111/j.1749-6632.2009.04416.x. PMID 19338508. S2CID 313685.
- Walsh, Neil P.; Halson, Shona L.; Sargent, Charli; Roach, Gregory D.; Nédélec, Mathieu; Gupta, Luke; Leeder, Jonathan; Fullagar, Hugh H.; Coutts, Aaron J.; Edwards, Ben J.; Pullinger, Samuel A.; Robertson, Colin M.; Burniston, Jatin G.; Lastella, Michele; Meur, Yann Le; Hausswirth, Christophe; Bender, Amy M.; Grandner, Michael A.; Samuels, Charles H. (1 April 2021). "Sleep and the athlete: narrative review and 2021 expert consensus recommendations". British Journal of Sports Medicine. 55 (7): 356–368. doi:10.1136/bjsports-2020-102025. ISSN 0306-3674. PMID 33144349.
- "5 Smart(ish) Things to Help You Wake Up Easier". Wirecutter: Reviews for the Real World. 17 February 2023. Retrieved 17 March 2023.
- Oh, Kyue Taek; Shin, Jaemyung; Kim, Jaejeung; Ko, Minsam (January 2020). "Analysis of a Wake-Up Task-Based Mobile Alarm App". Applied Sciences. 10 (11): 3993. doi:10.3390/app10113993. ISSN 2076-3417.
- Dotson, Noe (28 May 2015). "Puzzle Alarm Clock". Academic Excellence Showcase Schedule. Retrieved 17 March 2023.
- Olanoff, Drew (26 October 2011). "Mission Alarm Clock turns waking up into a game - The Next Web". TNW | Apps. Retrieved 17 March 2023.
- "Brain-cleaning sleeping cap gets US Army funding". New Atlas. 1 October 2021. Retrieved 17 March 2023.
- Bruni, Oliviero; Ferini-Strambi, Luigi; Giacomoni, Elena; Pellegrino, Paolo (February 2021). "Herbal Remedies and Their Possible Effect on the GABAergic System and Sleep". Nutrients. 13 (2): 530. doi:10.3390/nu13020530. ISSN 2072-6643. PMC 7914492. PMID 33561990.
- Bonilla, Diego A.; Moreno, Yurany; Gho, Camila; Petro, Jorge L.; Odriozola-Martínez, Adrián; Kreider, Richard B. (March 2021). "Effects of Ashwagandha (Withania somnifera) on Physical Performance: Systematic Review and Bayesian Meta-Analysis". Journal of Functional Morphology and Kinesiology. 6 (1): 20. doi:10.3390/jfmk6010020. ISSN 2411-5142. PMC 8006238. PMID 33670194.
- Lopresti, Adrian L.; Smith, Stephen J. (1 August 2021). "Ashwagandha (Withania somnifera) for the treatment and enhancement of mental and physical conditions: A systematic review of human trials". Journal of Herbal Medicine. 28: 100434. doi:10.1016/j.hermed.2021.100434. ISSN 2210-8033. S2CID 233958266.
- Gardiner, Carissa; Weakley, Jonathon; Burke, Louise M.; Roach, Gregory D.; Sargent, Charli; Maniar, Nirav; Townshend, Andrew; Halson, Shona L. (1 June 2023). "The effect of caffeine on subsequent sleep: A systematic review and meta-analysis". Sleep Medicine Reviews. 69: 101764. doi:10.1016/j.smrv.2023.101764. ISSN 1087-0792. PMID 36870101. S2CID 256645683.
- Halson, Shona L. (1 May 2014). "Sleep in Elite Athletes and Nutritional Interventions to Enhance Sleep". Sports Medicine. 44 (1): 13–23. doi:10.1007/s40279-014-0147-0. ISSN 1179-2035. PMC 4008810. PMID 24791913.
- Caddick, Zachary A.; Gregory, Kevin; Arsintescu, Lucia; Flynn-Evans, Erin E. (15 March 2018). "A review of the environmental parameters necessary for an optimal sleep environment". Building and Environment. 132: 11–20. doi:10.1016/j.buildenv.2018.01.020. ISSN 0360-1323.
- Buchner, Josef; Buntins, Katja; Kerres, Michael (February 2022). "The impact of augmented reality on cognitive load and performance: A systematic review". Journal of Computer Assisted Learning. 38 (1): 285–303. doi:10.1111/jcal.12617. ISSN 0266-4909. S2CID 243783802.
- Brunyé, Tad T.; Brou, Randy; Doty, Tracy Jill; Gregory, Frederick D.; Hussey, Erika K.; Lieberman, Harris R.; Loverro, Kari L.; Mezzacappa, Elizabeth S.; Neumeier, William H.; Patton, Debra J.; Soares, Jason W.; Thomas, Thaddeus P.; Yu, Alfred B. (1 December 2020). "A Review of US Army Research Contributing to Cognitive Enhancement in Military Contexts". Journal of Cognitive Enhancement. 4 (4): 453–468. doi:10.1007/s41465-020-00167-3. ISSN 2509-3304. S2CID 256621326.
- Lee, Yong-Seok; Silva, Alcino J. (February 2009). "The molecular and cellular biology of enhanced cognition". Nature Reviews Neuroscience. 10 (2): 126–140. doi:10.1038/nrn2572. ISSN 1471-0048. PMC 2664745. PMID 19153576.
- "George Church told us why he's listing "superhuman" gene hacks". Futurism. Retrieved 25 July 2021.
- "Protective alleles". arep.med.harvard.edu. Retrieved 25 July 2021.
- Eising, Else; Mirza-Schreiber, Nazanin; de Zeeuw, Eveline L.; Wang, Carol A.; Truong, Dongnhu T.; Allegrini, Andrea G.; Shapland, Chin Yang; Zhu, Gu; Wigg, Karen G.; Gerritse, Margot L.; et al. (30 August 2022). "Genome-wide analyses of individual differences in quantitatively assessed reading- and language-related skills in up to 34,000 people". Proceedings of the National Academy of Sciences. 119 (35): e2202764119. Bibcode:2022PNAS..11902764E. doi:10.1073/pnas.2202764119. ISSN 0027-8424. PMC 9436320. PMID 35998220.
- University press release: "Massive genome study informs the biology of reading and language". Max Planck Society via medicalxpress.com. Retrieved 18 September 2022.
- ""nootropic"[Title/Abstract] OR "smart drug"[Title/Abstract] OR "smart drugs"[Title/Abstract] OR "nootropics"[Title/Abstract] OR "cognitive enhancer"[Title/Abstract] - Search Results - PubMed". PubMed. Retrieved 20 March 2023.
- Lucke, Jayne C.; Bell, Stephanie K.; Patridge, Bradley J.; Hall, Wayne D. (2011). "Academic Doping or Viagra for the brain?". EMBO Rep. 12 (3): 197–201. doi:10.1038/embor.2011.15. PMC 3059919. PMID 21311560.
- Schuijer, Jantien W.; de Jong, Irja M.; Kupper, Frank; van Atteveldt, Nienke M. (2017). "Transcranial Electrical Stimulation to Enhance Cognitive Performance of Healthy Minors: A Complex Governance Challenge". Frontiers in Human Neuroscience. 11: 142. doi:10.3389/fnhum.2017.00142. ISSN 1662-5161. PMC 5366312. PMID 28396631.
- "The Ethics and Challenges Surrounding Neuroenhancement". NC State News. 8 May 2019. Retrieved 19 March 2023.
- "Doctor's Op-Ed: Consumers are losing trust in the supplements industry. Here's why we need to earn it back". Nutritional Outlook. September 2021. Retrieved 24 April 2022.
- "Prohibited, unlisted, even dangerous ingredients turn up in dietary supplements". Washington Post. Retrieved 24 April 2022.
- Eisenstein, Michael. "Setting Standards for Supplements". Scientific American. Retrieved 19 March 2023.
- Glisson, James K. (14 March 2011). "Dietary Supplements: Safety Issues and Quality Control". Archives of Internal Medicine. 171 (5): 476–7, author reply 477. doi:10.1001/archinternmed.2011.53. PMID 21403052.
- Long, Chiau Soon; Kumaran, Harshily; Goh, Khang Wen; Bakrin, Faizah Safina; Ming, Long Chiau; Rehman, Inayat Ur; Dhaliwal, Jagjit Singh; Hadi, Muhammad Abdul; Sim, Yee Wai; Tan, Ching Siang (April 2022). "Online Pharmacies Selling Prescription Drugs: Systematic Review". Pharmacy. 10 (2): 42. doi:10.3390/pharmacy10020042. ISSN 2226-4787. PMC 9031186. PMID 35448701.
- "Are Dietary Supplements Safe?". www.cancer.org. Retrieved 19 March 2023.
- El Azab, Noha F.; Abdelaal, Sarah H.; Hassan, Said A.; El-Kosasy, Amira M. (24 December 2022). "Dietary supplement mislabelling: case study on selected slimming products by developing a green isocratic HPLC method for their quality control". Scientific Reports. 12 (1): 22305. Bibcode:2022NatSR..1222305E. doi:10.1038/s41598-022-24830-1. ISSN 2045-2322. PMC 9790016. PMID 36566240.
- Grohn, Kristopher J.; Moyer, Brandon S.; Wortel, Danique C.; Fisher, Cheyanne M.; Lumen, Ellie; Bianchi, Anthony H.; Kelly, Kathleen; Campbell, Paul S.; Hagrman, Douglas E.; Bagg, Roger G.; Clement, James; Wolfe, Aaron J.; Basso, Andrea; Nicoletti, Cristina; Lai, Giovanni; Provinciali, Mauro; Malavolta, Marco; Moody, Kelsey J. (1 April 2021). "C60 in olive oil causes light-dependent toxicity and does not extend lifespan in mice". GeroScience. 43 (2): 579–591. doi:10.1007/s11357-020-00292-z. ISSN 2509-2723. PMC 8110650. PMID 33123847. S2CID 226206079.
- "Play It Safe When Choosing Vitamins and Supplements". www.southuniversity.edu. Retrieved 19 March 2023.
- Clausen, Angela; Schlueter, Kirsten (1 March 2017). "Motives for Using Food Supplements by Otherwise Healthy Adults: Historic and Current Perspectives with Special Focus on Germany". Health Behavior and Policy Review. 4 (2): 129–141. doi:10.14485/HBPR.4.2.4.
- Marois, Alexandre; Lafond, Daniel (1 November 2022). "Augmenting cognitive work: a review of cognitive enhancement methods and applications for operational domains". Cognition, Technology & Work. 24 (4): 589–608. doi:10.1007/s10111-022-00715-1. ISSN 1435-5566. S2CID 252372408.
- Jwa, Anita S. (2021). "Enhancing the developing brain: tensions between parent, child, and state in the United States". Journal of Law and the Biosciences. 8 (1): lsab017. doi:10.1093/jlb/lsab017. PMC 8223904. PMID 34188944.
- Saritas, Ozcan (2019). "Emerging Technologies, Trends and Wild Cards in Human Enhancement". Emerging Technologies for Economic Development. Science, Technology and Innovation Studies. Springer International Publishing. pp. 243–259. doi:10.1007/978-3-030-04370-4_11. ISBN 978-3-030-04370-4. S2CID 169759910.
- Maccioni, Ricardo Benjamin; Calfío, Camila; González, Andrea; Lüttges, Valentina (February 2022). "Novel Nutraceutical Compounds in Alzheimer Prevention". Biomolecules. 12 (2): 249. doi:10.3390/biom12020249. ISSN 2218-273X. PMC 8961630. PMID 35204750.
- Sattler, S.; Sauer, C.; Mehlkop, G.; Graeff, P. (2013). "The Rationale for Consuming Cognitive Enhancement Drugs in University Students and Teachers". PLOS ONE. 8 (7): e68821. Bibcode:2013PLoSO...868821S. doi:10.1371/journal.pone.0068821. PMC 3714277. PMID 23874778.
- Sattler, S.; Forlini, C.; Racine, E.; Sauer, C. (2013). "Impact of Contextual Factors and Substance Characteristics on Perspectives toward Cognitive Enhancement". PLOS ONE. 8 (8): e71452. Bibcode:2013PLoSO...871452S. doi:10.1371/journal.pone.0071452. PMC 3733969. PMID 23940757.
- Wiegel C.; Sattler S.; Göritz A. S. (2015). "Work-related stress and cognitive enhancement among university teachers". Anxiety, Stress & Coping. 29 (1): 1–18. doi:10.1080/10615806.2015.1025764. PMID 25747817. S2CID 22273733.
- Anomaly, Jonathan; Gyngell, Christopher; Savulescu, Julian (January 2020). "Great minds think different: Preserving cognitive diversity in an age of gene editing". Bioethics. 34 (1): 81–89. doi:10.1111/bioe.12585. ISSN 0269-9702. PMC 6973122. PMID 30941781.
- Gyngell, Chris; Easteal, Simon (January 2015). "Cognitive Diversity and Moral Enhancement". Cambridge Quarterly of Healthcare Ethics. 24 (1): 66–74. doi:10.1017/S0963180114000310. ISSN 0963-1801. PMID 25473859.
- Veit, Walter (1 December 2018). "Cognitive Enhancement and the Threat of Inequality". Journal of Cognitive Enhancement. 2 (4): 404–410. doi:10.1007/s41465-018-0108-x. ISSN 2509-3304. S2CID 256624542.
- Hernandez, Joe. "Billionaire Mark Cuban launches online pharmacy aimed at lowering generic drug prices". Retrieved 20 March 2023.
- "Online Pharmacies Can Help You Save Big on Prescription Drugs - Consumer Reports". 18 February 2023. Archived from the original on 2023-02-18. Retrieved 20 March 2023.
- Flower, R. (1 September 2012). "The Osler Lecture 2012 'Pharmacology 2.0, medicines, drugs and human enhancement'". QJM. 105 (9): 823–830. doi:10.1093/qjmed/hcs105. PMID 22723455.
- Boire, Part I
- Boire, Richard Glen (2000). "On Cognitive Liberty Part II". Journal of Cognitive Liberties. 1 (2). Archived from the original on 2017-02-10. Retrieved 2015-05-16.
- "Keeping Freedom in Mind". Center for Cognitive Liberty and Ethics. Archived from the original on 24 April 2018. Retrieved 3 May 2014.
- Blitz, 1058-1060
- Sententia (2013), 356
- Sententia (2013), 355-6
- Ienca, Marcello; Andorno, Roberto (26 April 2017). "Towards new human rights in the age of neuroscience and neurotechnology". Life Sciences, Society and Policy. 13 (1): 5. doi:10.1186/s40504-017-0050-1. ISSN 2195-7819. PMC 5447561. PMID 28444626.
- Explanatory article from one of its authors: Ienca, Marcello. "Do We Have a Right to Mental Privacy and Cognitive Liberty?". Scientific American Blog Network. Retrieved 20 March 2023.
- News article about the study: "Four new human rights proposed to protect us from mind reading and brain hacking". New Atlas. 28 April 2017. Retrieved 20 March 2023.
- Bublitz, Christoph (2015). "Cognitive Liberty or the International Human Right to Freedom of Thought". Handbook of Neuroethics. Springer Netherlands. pp. 1309–1333. ISBN 978-94-007-4707-4.
- Corbyn, Zoë (4 March 2023). "Prof Nita Farahany: 'We need a new human right to cognitive liberty'". The Observer. Retrieved 10 March 2023.
- "Tech that aims to read your mind and probe your memories is already here". MIT Technology Review. Retrieved 20 March 2023.
- Muñoz, José M. (17 March 2023). "Achieving cognitive liberty The Battle for Your Brain: Defending the Right to Think Freely in the Age of Neurotechnology Nita A. Farahany St. Martin's Press, 2023. 288 pp". Science. 379 (6637): 1097. doi:10.1126/science.adf8306. PMID 36927033. S2CID 257558736. Retrieved 20 March 2023.
- "In the face of neurotechnology advances, Chile passes 'neuro rights' law". techxplore.com. Retrieved 26 January 2022.
- Zwart H. (2014). "Limitless as a neuro-pharmaceutical experiment and as a Daseinsanalyse: on the use of fiction in preparatory debates on cognitive enhancement" (PDF). Medicine, Health Care and Philosophy. 17 (1): 29–38. doi:10.1007/s11019-013-9481-5. PMID 23585022. S2CID 29893291.
- Antal, Andrea; Luber, Bruce; Brem, Anna-Katharine; Bikson, Marom; Brunoni, Andre R.; Cohen Kadosh, Roi; Dubljević, Veljko; Fecteau, Shirley; Ferreri, Florinda; Flöel, Agnes; Hallett, Mark; Hamilton, Roy H.; Herrmann, Christoph S.; Lavidor, Michal; Loo, Collen; Lustenberger, Caroline; Machado, Sergio; Miniussi, Carlo; Moliadze, Vera; Nitsche, Michael A; Rossi, Simone; Rossini, Paolo M.; Santarnecchi, Emiliano; Seeck, Margitta; Thut, Gregor; Turi, Zsolt; Ugawa, Yoshikazu; Venkatasubramanian, Ganesan; Wenderoth, Nicole; Wexler, Anna; Ziemann, Ulf; Paulus, Walter (1 January 2022). "Non-invasive brain stimulation and neuroenhancement". Clinical Neurophysiology Practice. 7: 146–165. doi:10.1016/j.cnp.2022.05.002. ISSN 2467-981X. PMC 9207555. PMID 35734582.
- Garmany, Armin; Yamada, Satsuki; Terzic, Andre (23 September 2021). "Longevity leap: mind the healthspan gap". npj Regenerative Medicine. 6 (1): 57. doi:10.1038/s41536-021-00169-5. ISSN 2057-3995. PMC 8460831. PMID 34556664.
- Non-profit hospital press release: Buckles, Susan. "A regenerative reset for aging » Center for Regenerative Biotherapeutics". Mayo Clinic. Retrieved 1 March 2023.
- Wexler, Anna (2017). "The Social Context of "Do-It-Yourself" Brain Stimulation: Neurohackers, Biohackers, and Lifehackers". Frontiers in Human Neuroscience. 11: 224. doi:10.3389/fnhum.2017.00224. ISSN 1662-5161. PMC 5423946. PMID 28539877.
- Onaolapo, Adejoke Yetunde; Obelawo, Adebimpe Yemisi; Onaolapo, Olakunle James (May 2019). "Brain Ageing, Cognition and Diet: A Review of the Emerging Roles of Food-Based Nootropics in Mitigating Age-Related Memory Decline". Current Aging Science. 12 (1): 2–14. doi:10.2174/1874609812666190311160754. ISSN 1874-6098. PMC 6971896. PMID 30864515.
- Katz, Sylvan. "Forum: Roses are black, violets are green - The emergence of amateur genetic engineers". New Scientist. Retrieved 2020-04-03.
- Knapton, Sarah (2019-11-02). "Neurohacking cream which helps you learn guitar faster available in five years". The Telegraph. ISSN 0307-1235. Retrieved 2020-04-03.
- Health, Center for Devices and Radiological (2019-02-09). "Vercise Deep Brain Stimulation (DBS) System - P150031". FDA.
- Wexler, Anna (10 May 2017). "The Social Context of "Do-It-Yourself" Brain Stimulation: Neurohackers, Biohackers, and Lifehackers". Frontiers in Human Neuroscience. 11: 224. doi:10.3389/fnhum.2017.00224. ISSN 1662-5161. PMC 5423946. PMID 28539877.
- Adams, B. L. M. (2019). Monitoring the internet for nootropic substances available to Australian consumers (thesis). University of Tasmania. doi:10.25959/23238149.v1. Retrieved 19 March 2023.
- Squirrell, Tim (September 2019). "Platform dialectics: The relationships between volunteer moderators and end users on reddit". New Media & Society. 21 (9): 1910–1927. doi:10.1177/1461444819834317. ISSN 1461-4448. S2CID 151084817.
- Natter, Johan; Michel, Bruno (September 2020). "Memantine misuse and social networks: A content analysis of Internet self-reports". Pharmacoepidemiology and Drug Safety. 29 (9): 1189–1193. doi:10.1002/pds.5070. ISSN 1053-8569. PMID 32602152. S2CID 220270495.
- Eickenhorst, Patrick; Vitzthum, Karin; Klapp, Burghard F.; Groneberg, David; Mache, Stefanie (2012). "Neuroenhancement Among German University Students: Motives, Expectations, and Relationship with Psychoactive Lifestyle Drugs". Journal of Psychoactive Drugs. 44 (5): 418–427. doi:10.1080/02791072.2012.736845. PMID 23457893. S2CID 6621896.
- Sattler S.; Wiegel C. (2013). "Cognitive test anxiety and cognitive enhancement: the influence of students' worries on their use of performance-enhancing drugs". Substance Use and Misuse. 48 (3): 220–32. doi:10.3109/10826084.2012.751426. PMID 23302063. S2CID 34698382.
- Sattler S.; Sauer C.; Mehlkop G.; Graeff P. (2013). "The Rationale for Consuming Cognitive Enhancement Drugs in University Students and Teachers". PLOS ONE. 8 (7): e68821. Bibcode:2013PLoSO...868821S. doi:10.1371/journal.pone.0068821. PMC 3714277. PMID 23874778.
- Sattler S.; Schunck R. (2016). "Associations Between the Big Five Personality Traits and the Non-Medical Use of Prescription Drugs for Cognitive Enhancement". Frontiers in Psychology. 6: 1971. doi:10.3389/fpsyg.2015.01971. PMC 4700267. PMID 26779083.
- Sattler, S.; Mehlkop, G.; Graeff, P.; Sauer, C. (2014). "Evaluating the drivers of and obstacles to the willingness to use cognitive enhancement drugs: the influence of drug characteristics, social environment, and personal characteristics". Substance Abuse Treatment, Prevention, and Policy. 9: 8. doi:10.1186/1747-597X-9-8. PMC 3928621. PMID 24484640.
- Olsen, Nicole (2013). "Caffeine Consumption Habits and Perceptions among University of New Hampshire Students". Honors Theses.
Further reading
- Blank, Robert H. (5 November 2016). Cognitive Enhancement: Social and Public Policy Issues. Palgrave Macmillan UK. ISBN 978-1-349-84797-6.
- Bostrom, Nick; Sandberg, Anders (2009). "Cognitive Enhancement: Methods, Ethics, Regulatory Challenges". Science and Engineering Ethics. 15 (3): 311–341. doi:10.1007/s11948-009-9142-5. PMID 19543814. S2CID 6846531.
- Veit, Walter (2018). "Cognitive Enhancement and the Threat of Inequality". Journal of Cognitive Enhancement. 2 (4): 404–410. doi:10.1007/s41465-018-0108-x. S2CID 158643005.