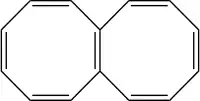
Octalene
Octalene is a polycyclic hydrocarbon composed of two fused cyclooctatetraene rings.[1]
 | |
| Names | |
|---|---|
| IUPAC name
Octalene | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| EC Number |
|
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C14H12 | |
| Molar mass | 180.250 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Anions
Octalene can be readily reduced by lithium to a dianion C14H2−12 and, unusually for such a small molecule, a tetraanion C14H4−12.[2] The di-anion has its two negative charges in one ring, converting that ring into a 10-pi electron aromatic system similar to the di-anion of cyclooctatetraene. In the 18-pi electron tetra-anion, both rings effectively have access to 10 pi electrons, leading to a planar, bicyclic aromatic structure analogous to that of naphthalene.
References
- Koseki, S.; Kataoka, M.; Hanamura, M.; Nakajima, T.; Toyota, A. (1984). "Theoretical studies on octalene: the planar and nonplanar structures and the isomerization reactions among the nonplanar structures". The Journal of Organic Chemistry. 49 (16): 2988–2993. doi:10.1021/jo00190a026.
- Müllen, K., Oth, J. F. M., Engels, H.-W. and Vogel, E. (1979), Dianion and Tetraanion Octalene. Angew. Chem. Int. Ed. Engl., 18: 229–231. doi:10.1002/anie.197902291
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.