Propalene
Propalene or bicyclo[1.1.0]buta-1,3-diene is a polycyclic hydrocarbon composed of two fused cyclopropene rings.[1][2] Computational studies indicate that the molecule is planar, with the carbon framework forming a parallelogram that has distinctly alternating short and long carbon–carbon bonds.[3]
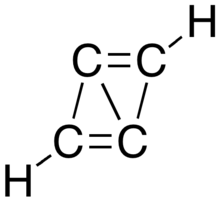 | |
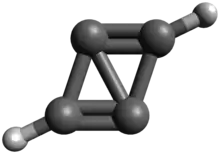 | |
| Names | |
|---|---|
| Preferred IUPAC name
Bicyclo[1.1.0]buta-1,3-diene | |
| Other names
Propalene, Bicyclobutadiene | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C4H2 | |
| Molar mass | 50.060 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
References
- Toyota, Azumao; Nakajima, Takeshi (1979). "The nonsinglet instabilities of the hartree-fock solutions for nonalternant hydrocarbons". Theoretica Chimica Acta. 53 (4): 297–308. doi:10.1007/BF00555689.
- D. P. Craig (1951). "cycloButadiene and some other pseudoaromatic compounds". J. Chem. Soc.: 3175–3182. doi:10.1039/JR9510003175.
- Koseki, Shiro; Toyota, Azumao; Muramatsu, Takasi; Asada, Toshio; Matsunaga, Nikita (2016). "Numerical Estimation of the Pseudo-Jahn–Teller Effect Using Nonadiabatic Coupling Integrals in Monocyclic and Bicyclic Conjugated Molecules". Journal of Physical Chemistry A. 120 (51): 10207–10215. Bibcode:2016JPCA..12010207K. doi:10.1021/acs.jpca.6b09632.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.