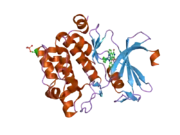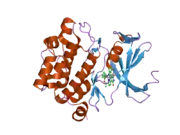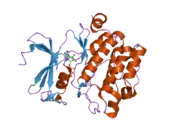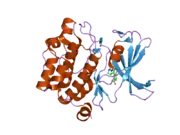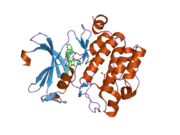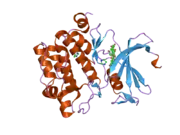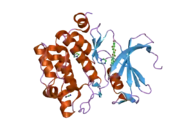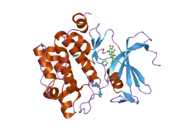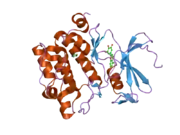PIM1
Proto-oncogene serine/threonine-protein kinase Pim-1 is an enzyme that in humans is encoded by the PIM1 gene.[5][6][7]
Pim-1 is a proto-oncogene which encodes for the serine/threonine kinase of the same name. The pim-1 oncogene was first described in relation to murine T-cell lymphomas, as it was the locus most frequently activated by the Moloney murine leukemia virus.[8] Subsequently, the oncogene has been implicated in multiple human cancers, including prostate cancer, acute myeloid leukemia and other hematopoietic malignancies.[9] Primarily expressed in spleen, thymus, bone marrow, prostate, oral epithelial, hippocampus and fetal liver cells, Pim-1 has also been found to be highly expressed in cell cultures isolated from human tumors.[8] Pim-1 is mainly involved in cell cycle progression, apoptosis and transcriptional activation, as well as more general signal transduction pathways.[8] Pim-1's role in oncogenic signalling has led to it becoming a widely studied target in cancer research, with numerous drug candidates under investigation which target it.[10][11]
Gene
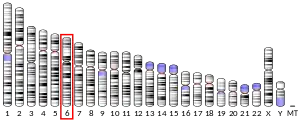
Located on chromosome 6 (6p21.2), the gene encompasses 5Kb of DNA, including 6 exons and 5 introns. Expression of Pim-1 has been shown to be regulated by the JAK/STAT pathway. Direct binding of transcription factors STAT3 and STAT5 to the Pim-1 promoter results in the transcription of Pim-1.[8] The Pim-1 gene has been found to be conserved in dogs, cows, mice, rats, zebrafish and C. elegans. Pim-1 deficient mice have been shown to be phenotypically normal, indicating that there is redundancy in the function of this kinase.[8] In fact, sequence homology searches have shown that two other Pim-1-like kinases, Pim-2 and Pim-3, are structurally and functionally similar.[8] The Pim-1 gene encodes has multiple translation initiation sites, resulting in two proteins of 34 and 44kD.[8]
Protein structure
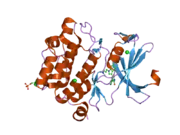
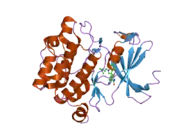
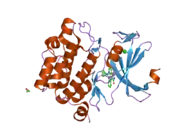
Human, murine and rat Pim-1 contain 313 amino acids, and have a 94 – 97% amino acid identity.[8] The active site of the protein, ranging from amino acids 38-290, is composed of several conserved motifs, including a glycine loop motif, a phosphate binding site and a proton acceptor site.[8] Modification of the protein at amino acid 67 (lysine to methionine) results in the inactivation of the kinase.[8]
Activation and stabilization
Pim-1 is primarily involved in cytokine signaling, and has been implicated in many signal transduction pathways. Because Pim-1 transcription is initiated by STAT3 and STAT5, its production is regulated by the cytokines that regulate the STAT pathway, or STAT factors. These include interleukins (IL-2, IL-3,IL-5, IL-6, IL-7, IL12, IL-15), prolactin, TNFα, EGF and IFNγ, among others.[8] Pim-1 itself can bind to negative regulators of the JAK/STAT pathway, resulting in a negative feedback loop.
Although little is known about the post-transcriptional modifications of Pim-1, it has been hypothesized that Hsp90 is responsible for the folding and stabilization of Pim-1, although the exact mechanism has yet to be discovered.[8] Furthermore, the serine/threonine phosphatase PP2 has been shown to degrade Pim-1.
Interactions
PIM1 has been shown to interact with:
- CBX3,[12]
- CDC25A,[13]
- Heat shock protein 90kDa alpha (cytosolic), member A1,[14]
- NFATC1,[15]
- Nuclear mitotic apparatus protein 1,[16]
- P21,[17]
- SND1[18] and
- RELA.[19]
Other known substrates/binding partners of Pim-1 include proteins involved in transcription regulation (nuclear adaptor protein p100, HP-1, PAP-1 and TRAF2 / SNX6), and regulation of the JAK/STAT pathway (SOCS1 and SOCS3).[8] Furthermore, Pim-1 has been shown to be a cofactor for c-Myc, a transcription factor believed to regulate 15% of all genes, and their synergy has been in prostate tumorigenesis.[20]
Pim-1 is able to phosphorylate many targets, including itself. Many of its targets are involved in cell cycle regulation.
Activates
Clinical implications
Pim-1 is directly involved in the regulation of cell cycle progression and apoptosis, and has been implicated in numerous cancers including prostate cancer, Burkitt's lymphoma and oral cancer, as well as numerous hematopoietic lymphomas. Single nucleotide polymorphisms in the Pim-1 gene have been associated with increased risk for lung cancer in Korean patients, and have also been found in diffuse large cell lymphomas.[21] As well as showing useful activity against a range of cancers,[11] PIM kinase inhibitors have also been suggested as possible treatments for Alzheimer's disease.[22] PIM expression is sufficient to drive resistance to anti-angiogenic agents in prostate and colon cancer models, although the mechanism is not fully elucidated.[23] It has been suggested that a co-targeted therapeutic approach to inhibition of Pim-1 in cancer may be preferable, with suggested co-targets including the PI3K pathway and more.[10] PIM1 expression was found to be elevated during aging and to contribute to the development of pulmonary fibrosis.[24]
Inhibitors
A large number of small molecule inhibitors of PIM1 have been developed. Clinical trial results so far have showed promising anti-cancer activity, but side effects due to insufficient selectivity have proved problematic and research continues to find more potent and selective inhibitors for this target.[25][26][27][28][29][30][31][10][11]
- Examples
References
- GRCh38: Ensembl release 89: ENSG00000137193 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000024014 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Entrez Gene: PIM1 pim-1 oncogene".
- Domen J, Von Lindern M, Hermans A, Breuer M, Grosveld G, Berns A (June 1987). "Comparison of the human and mouse PIM-1 cDNAs: nucleotide sequence and immunological identification of the in vitro synthesized PIM-1 protein". Oncogene Research. 1 (1): 103–12. PMID 3329709.
- Meeker TC, Nagarajan L, ar-Rushdi A, Rovera G, Huebner K, Croce CM (June 1987). "Characterization of the human PIM-1 gene: a putative proto-oncogene coding for a tissue specific member of the protein kinase family". Oncogene Research. 1 (1): 87–101. PMID 3329711.
- Bachmann M, Möröy T (April 2005). "The serine/threonine kinase Pim-1". The International Journal of Biochemistry & Cell Biology. 37 (4): 726–30. doi:10.1016/j.biocel.2004.11.005. PMID 15694833.
- "Pim-1 Oncogene". Retrieved 2015-12-14.
- Luszczak S, Kumar C, Sathyadevan VK, Simpson BS, Gately KA, Whitaker HC, Heavey S (2020). "PIM kinase inhibition: co-targeted therapeutic approaches in prostate cancer". Signal Transduction and Targeted Therapy. 5: 7. doi:10.1038/s41392-020-0109-y. PMC 6992635. PMID 32025342.
- Malone T, Schäfer L, Simon N, Heavey S, Cuffe S, Finn S, et al. (March 2020). "Current perspectives on targeting PIM kinases to overcome mechanisms of drug resistance and immune evasion in cancer" (PDF). Pharmacology & Therapeutics. 207: 107454. doi:10.1016/j.pharmthera.2019.107454. PMID 31836451. S2CID 209357486.
- Koike N, Maita H, Taira T, Ariga H, Iguchi-Ariga SM (February 2000). "Identification of heterochromatin protein 1 (HP1) as a phosphorylation target by Pim-1 kinase and the effect of phosphorylation on the transcriptional repression function of HP1(1)". FEBS Letters. 467 (1): 17–21. doi:10.1016/S0014-5793(00)01105-4. PMID 10664448. S2CID 29392124.
- Mochizuki T, Kitanaka C, Noguchi K, Muramatsu T, Asai A, Kuchino Y (June 1999). "Physical and functional interactions between Pim-1 kinase and Cdc25A phosphatase. Implications for the Pim-1-mediated activation of the c-Myc signaling pathway". The Journal of Biological Chemistry. 274 (26): 18659–66. doi:10.1074/jbc.274.26.18659. PMID 10373478.
- Mizuno K, Shirogane T, Shinohara A, Iwamatsu A, Hibi M, Hirano T (March 2001). "Regulation of Pim-1 by Hsp90". Biochemical and Biophysical Research Communications. 281 (3): 663–9. doi:10.1006/bbrc.2001.4405. PMID 11237709.
- Rainio EM, Sandholm J, Koskinen PJ (February 2002). "Cutting edge: Transcriptional activity of NFATc1 is enhanced by the Pim-1 kinase". Journal of Immunology. 168 (4): 1524–7. doi:10.4049/jimmunol.168.4.1524. PMID 11823475.
- Bhattacharya N, Wang Z, Davitt C, McKenzie IF, Xing PX, Magnuson NS (July 2002). "Pim-1 associates with protein complexes necessary for mitosis". Chromosoma. 111 (2): 80–95. doi:10.1007/s00412-002-0192-6. PMID 12111331. S2CID 26016943.
- Wang Z, Bhattacharya N, Mixter PF, Wei W, Sedivy J, Magnuson NS (December 2002). "Phosphorylation of the cell cycle inhibitor p21Cip1/WAF1 by Pim-1 kinase". Biochimica et Biophysica Acta (BBA) - Molecular Cell Research. 1593 (1): 45–55. doi:10.1016/S0167-4889(02)00347-6. PMID 12431783.
- Leverson JD, Koskinen PJ, Orrico FC, Rainio EM, Jalkanen KJ, Dash AB, Eisenman RN, Ness SA (October 1998). "Pim-1 kinase and p100 cooperate to enhance c-Myb activity". Molecular Cell. 2 (4): 417–25. doi:10.1016/S1097-2765(00)80141-0. PMID 9809063.
- Nihira K, Ando Y, Yamaguchi T, Kagami Y, Miki Y, Yoshida K (April 2010). "Pim-1 controls NF-kappaB signalling by stabilizing RelA/p65". Cell Death and Differentiation. 17 (4): 689–98. doi:10.1038/cdd.2009.174. PMID 19911008.
- Wang J, Kim J, Roh M, Franco OE, Hayward SW, Wills ML, Abdulkadir SA (April 2010). "Pim1 kinase synergizes with c-MYC to induce advanced prostate carcinoma". Oncogene. 29 (17): 2477–87. doi:10.1038/onc.2010.10. PMC 2861731. PMID 20140016.
- Kim DS, Sung JS, Shin ES, Ryu JS, Choi IK, Park KH, Park Y, Kim EB, Park SJ, Kim YH (December 2008). "Association of single nucleotide polymorphisms in PIM-1 gene with the risk of Korean lung cancer". Cancer Research and Treatment. 40 (4): 190–6. doi:10.4143/crt.2008.40.4.190. PMC 2697471. PMID 19688129.
- Velazquez R, Shaw DM, Caccamo A, Oddo S (July 2016). "Pim1 inhibition as a novel therapeutic strategy for Alzheimer's disease". Molecular Neurodegeneration. 11 (1): 52. doi:10.1186/s13024-016-0118-z. PMC 4944476. PMID 27412291.
- Casillas AL, Toth RK, Sainz AG, Singh N, Desai AA, Kraft AS, Warfel NA (2018). "Hypoxia-Inducible PIM Kinase Expression Promotes Resistance to Antiangiogenic Agents". Clinical Cancer Research. 24 (1): 169–180. doi:10.1158/1078-0432.CCR-17-1318. PMC 6214353. PMID 29084916.
- Pham TX, Lee J, Guan J, Caporarello N, Meridew JA, Jones DL, et al. (February 2022). "Transcriptional analysis of lung fibroblasts identifies PIM1 signaling as a driver of aging-associated persistent fibrosis". JCI Insight. 7 (6). doi:10.1172/jci.insight.153672. PMC 8986080. PMID 35167499.
- Morwick T (February 2010). "Pim kinase inhibitors: a survey of the patent literature". Expert Opinion on Therapeutic Patents. 20 (2): 193–212. doi:10.1517/13543770903496442. PMID 20100002. S2CID 19401237.
- Merkel AL, Meggers E, Ocker M (April 2012). "PIM1 kinase as a target for cancer therapy". Expert Opinion on Investigational Drugs. 21 (4): 425–36. doi:10.1517/13543784.2012.668527. PMID 22385334. S2CID 26602099.
- Foulks JM, Carpenter KJ, Luo B, Xu Y, Senina A, Nix R, Chan A, Clifford A, Wilkes M, Vollmer D, Brenning B, Merx S, Lai S, McCullar MV, Ho KK, Albertson DJ, Call LT, Bearss JJ, Tripp S, Liu T, Stephens BJ, Mollard A, Warner SL, Bearss DJ, Kanner SB (May 2014). "A small-molecule inhibitor of PIM kinases as a potential treatment for urothelial carcinomas". Neoplasia. 16 (5): 403–412. doi:10.1016/j.neo.2014.05.004. PMC 4198696. PMID 24953177.
- Arunesh GM, Shanthi E, Krishna MH, Sooriya Kumar J, Viswanadhan VN (January 2014). "Small molecule inhibitors of PIM1 kinase: July 2009 to February 2013 patent update". Expert Opinion on Therapeutic Patents. 24 (1): 5–17. doi:10.1517/13543776.2014.848196. PMID 24131033. S2CID 2331769.
- Keane NA, Reidy M, Natoni A, Raab MS, O'Dwyer M (July 2015). "Targeting the Pim kinases in multiple myeloma". Blood Cancer Journal. 5 (7): e325. doi:10.1038/bcj.2015.46. PMC 4526774. PMID 26186558.
- Le BT, Kumarasiri M, Adams JR, Yu M, Milne R, Sykes MJ, Wang S (2015). "Targeting Pim kinases for cancer treatment: opportunities and challenges". Future Medicinal Chemistry. 7 (1): 35–53. doi:10.4155/fmc.14.145. PMID 25582332.
- Tursynbay Y, Zhang J, Li Z, Tokay T, Zhumadilov Z, Wu D, Xie Y (February 2016). "Pim-1 kinase as cancer drug target: An update". Biomedical Reports. 4 (2): 140–146. doi:10.3892/br.2015.561. PMC 4734217. PMID 26893828.
- Keeton EK, McEachern K, Dillman KS, Palakurthi S, Cao Y, Grondine MR, et al. (February 2014). "AZD1208, a potent and selective pan-Pim kinase inhibitor, demonstrates efficacy in preclinical models of acute myeloid leukemia". Blood. 123 (6): 905–13. doi:10.1182/blood-2013-04-495366. PMC 3916880. PMID 24363397.
- Burger MT, Nishiguchi G, Han W, Lan J, Simmons R, Atallah G, et al. (November 2015). "Identification of N-(4-((1R,3S,5S)-3-Amino-5-methylcyclohexyl)pyridin-3-yl)-6-(2,6-difluorophenyl)-5-fluoropicolinamide (PIM447), a Potent and Selective Proviral Insertion Site of Moloney Murine Leukemia (PIM) 1, 2, and 3 Kinase Inhibitor in Clinical Trials for Hematological Malignancies". Journal of Medicinal Chemistry. 58 (21): 8373–86. doi:10.1021/acs.jmedchem.5b01275. PMID 26505898.
- Mumenthaler SM, Ng PY, Hodge A, Bearss D, Berk G, Kanekal S, et al. (October 2009). "Pharmacologic inhibition of Pim kinases alters prostate cancer cell growth and resensitizes chemoresistant cells to taxanes". Molecular Cancer Therapeutics. 8 (10): 2882–93. doi:10.1158/1535-7163.MCT-09-0293. PMC 2808126. PMID 19825806.
- Chen LS, Redkar S, Taverna P, Cortes JE, Gandhi V (July 2011). "Mechanisms of cytotoxicity to Pim kinase inhibitor, SGI-1776, in acute myeloid leukemia". Blood. 118 (3): 693–702. doi:10.1182/blood-2010-12-323022. PMC 3142906. PMID 21628411.
- Foulks JM, Carpenter KJ, Luo B, Xu Y, Senina A, Nix R, et al. (May 2014). "A small-molecule inhibitor of PIM kinases as a potential treatment for urothelial carcinomas". Neoplasia. 16 (5): 403–12. doi:10.1016/j.neo.2014.05.004. PMC 4198696. PMID 24953177.
Further reading
- Ragoussis J, Senger G, Mockridge I, Sanseau P, Ruddy S, Dudley K, Sheer D, Trowsdale J (November 1992). "A testis-expressed Zn finger gene (ZNF76) in human 6p21.3 centromeric to the MHC is closely linked to the human homolog of the t-complex gene tcp-11". Genomics. 14 (3): 673–9. doi:10.1016/S0888-7543(05)80167-3. PMID 1427894.
- Saris CJ, Domen J, Berns A (March 1991). "The pim-1 oncogene encodes two related protein-serine/threonine kinases by alternative initiation at AUG and CUG". The EMBO Journal. 10 (3): 655–64. doi:10.1002/j.1460-2075.1991.tb07994.x. PMC 452698. PMID 1825810.
- Reeves R, Spies GA, Kiefer M, Barr PJ, Power M (June 1990). "Primary structure of the putative human oncogene, pim-1". Gene. 90 (2): 303–7. doi:10.1016/0378-1119(90)90195-W. PMID 2205533.
- Amson R, Sigaux F, Przedborski S, Flandrin G, Givol D, Telerman A (November 1989). "The human protooncogene product p33pim is expressed during fetal hematopoiesis and in diverse leukemias". Proceedings of the National Academy of Sciences of the United States of America. 86 (22): 8857–61. doi:10.1073/pnas.86.22.8857. PMC 298389. PMID 2682662.
- Telerman A, Amson R, Zakut-Houri R, Givol D (April 1988). "Identification of the human pim-1 gene product as a 33-kilodalton cytoplasmic protein with tyrosine kinase activity". Molecular and Cellular Biology. 8 (4): 1498–503. doi:10.1128/mcb.8.4.1498. PMC 363308. PMID 2837645.
- Meeker TC, Nagarajan L, ar-Rushdi A, Croce CM (October 1987). "Cloning and characterization of the human PIM-1 gene: a putative oncogene related to the protein kinases". Journal of Cellular Biochemistry. 35 (2): 105–12. doi:10.1002/jcb.240350204. PMID 3429489. S2CID 43495337.
- Zakut-Houri R, Hazum S, Givol D, Telerman A (1987). "The cDNA sequence and gene analysis of the human pim oncogene". Gene. 54 (1): 105–11. doi:10.1016/0378-1119(87)90352-0. PMID 3475233.
- Leverson JD, Koskinen PJ, Orrico FC, Rainio EM, Jalkanen KJ, Dash AB, Eisenman RN, Ness SA (October 1998). "Pim-1 kinase and p100 cooperate to enhance c-Myb activity". Molecular Cell. 2 (4): 417–25. doi:10.1016/S1097-2765(00)80141-0. PMID 9809063.
- Mochizuki T, Kitanaka C, Noguchi K, Muramatsu T, Asai A, Kuchino Y (June 1999). "Physical and functional interactions between Pim-1 kinase and Cdc25A phosphatase. Implications for the Pim-1-mediated activation of the c-Myc signaling pathway". The Journal of Biological Chemistry. 274 (26): 18659–66. doi:10.1074/jbc.274.26.18659. PMID 10373478.
- Koike N, Maita H, Taira T, Ariga H, Iguchi-Ariga SM (February 2000). "Identification of heterochromatin protein 1 (HP1) as a phosphorylation target by Pim-1 kinase and the effect of phosphorylation on the transcriptional repression function of HP1(1)". FEBS Letters. 467 (1): 17–21. doi:10.1016/S0014-5793(00)01105-4. PMID 10664448. S2CID 29392124.
- Maita H, Harada Y, Nagakubo D, Kitaura H, Ikeda M, Tamai K, Takahashi K, Ariga H, Iguchi-Ariga SM (August 2000). "PAP-1, a novel target protein of phosphorylation by pim-1 kinase". European Journal of Biochemistry. 267 (16): 5168–78. doi:10.1046/j.1432-1327.2000.01585.x. PMID 10931201.
- Mizuno K, Shirogane T, Shinohara A, Iwamatsu A, Hibi M, Hirano T (March 2001). "Regulation of Pim-1 by Hsp90". Biochemical and Biophysical Research Communications. 281 (3): 663–9. doi:10.1006/bbrc.2001.4405. PMID 11237709.
- Parks WT, Frank DB, Huff C, Renfrew Haft C, Martin J, Meng X, de Caestecker MP, McNally JG, Reddi A, Taylor SI, Roberts AB, Wang T, Lechleider RJ (June 2001). "Sorting nexin 6, a novel SNX, interacts with the transforming growth factor-beta family of receptor serine-threonine kinases". The Journal of Biological Chemistry. 276 (22): 19332–9. doi:10.1074/jbc.M100606200. PMID 11279102.
- Wang Z, Bhattacharya N, Meyer MK, Seimiya H, Tsuruo T, Tonani JA, Magnuson NS (June 2001). "Pim-1 negatively regulates the activity of PTP-U2S phosphatase and influences terminal differentiation and apoptosis of monoblastoid leukemia cells". Archives of Biochemistry and Biophysics. 390 (1): 9–18. doi:10.1006/abbi.2001.2370. PMID 11368509.
- Pasqualucci L, Neumeister P, Goossens T, Nanjangud G, Chaganti RS, Küppers R, Dalla-Favera R (July 2001). "Hypermutation of multiple proto-oncogenes in B-cell diffuse large-cell lymphomas". Nature. 412 (6844): 341–6. doi:10.1038/35085588. PMID 11460166. S2CID 4373198.
- Ishibashi Y, Maita H, Yano M, Koike N, Tamai K, Ariga H, Iguchi-Ariga SM (September 2001). "Pim-1 translocates sorting nexin 6/TRAF4-associated factor 2 from cytoplasm to nucleus". FEBS Letters. 506 (1): 33–8. doi:10.1016/S0014-5793(01)02881-2. PMID 11591366. S2CID 40248629.
- Rainio EM, Sandholm J, Koskinen PJ (February 2002). "Cutting edge: Transcriptional activity of NFATc1 is enhanced by the Pim-1 kinase". Journal of Immunology. 168 (4): 1524–7. doi:10.4049/jimmunol.168.4.1524. PMID 11823475.
- Nieborowska-Skorska M, Hoser G, Kossev P, Wasik MA, Skorski T (June 2002). "Complementary functions of the antiapoptotic protein A1 and serine/threonine kinase pim-1 in the BCR/ABL-mediated leukemogenesis". Blood. 99 (12): 4531–9. doi:10.1182/blood.V99.12.4531. PMID 12036885.
- Bhattacharya N, Wang Z, Davitt C, McKenzie IF, Xing PX, Magnuson NS (July 2002). "Pim-1 associates with protein complexes necessary for mitosis". Chromosoma. 111 (2): 80–95. doi:10.1007/s00412-002-0192-6. PMID 12111331. S2CID 26016943.
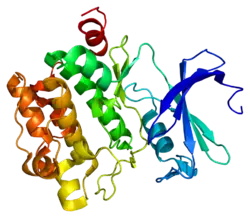


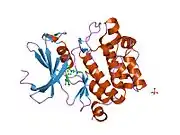
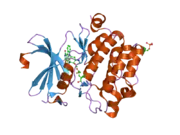
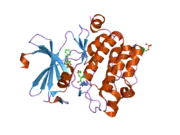
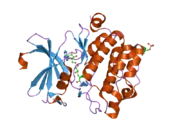
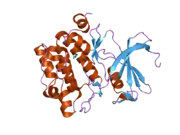
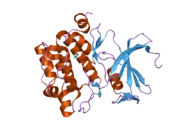
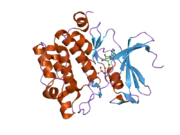
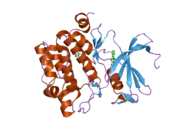
![1yxx: Crystal Structure of Kinase Pim1 in complex with (3E)-3-[(4-HYDROXYPHENYL)IMINO]-1H-INDOL-2(3H)-ONE](../I/PDB_1yxx_EBI.png.webp)