DNA polymerase alpha catalytic subunit
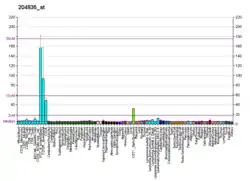
DNA polymerase alpha catalytic subunit is an enzyme that in humans is encoded by the POLA1 gene.[5]
Function
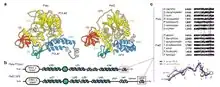
This gene encodes the p180 catalytic subunit of DNA polymerase α-primase. Pol α has limited processivity and lacks 3′ exonuclease activity for proofreading errors. Thus it is not well suited to efficiently and accurately copy long templates (unlike Pol Delta and Epsilon). Instead it plays a more limited role in replication. Pol α is responsible for the initiation of DNA replication at origins of replication (on both the leading and lagging strands) and during synthesis of Okazaki fragments on the lagging strand. The Pol α complex (pol α-DNA primase complex) consists of four subunits: the catalytic subunit POLA1, the regulatory subunit POLA2, and the small and the large primase subunits PRIM1 and PRIM2 respectively. Once primase has created the RNA primer, Pol α starts replication elongating the primer with ~20 nucleotides.
Clinical significance
In addition to its role during DNA replication, POLA1 plays a role in type I interferon activation. The POLA1 gene was found to be the site of a mutation resulting in X-linked reticulate pigmentary disorder (XLPDR), OMIM 301220). This leads to altered mRNA splicing and decreased expression of POLA1 protein to a level that does not impair DNA replication. The reduction in POLA1 expression is accompanied by marked reduction in cytosolic RNA:DNA hybrid molecules and a concomitant hyperactivation of the IRF3 pathway, with consequent overproduction of type I interferons.[7]
Moreover, POLA1 deficiency, typical for XLPDR, also impair direct cytotoxicity of NK cells. POLA1 inhibition or a natural deficiency (XLPDR) affects the way the lytic granules secreted toward target cells. As a result, NK cells in XLPDR patients display functional deficiency. Interestingly, the POLA1 deficiency typical for XLPDR is not associated with any genomic damages or cell cycle arrest.[8][9]
While the XLPDR mutation is resided in intron 13th, other somatic mutations in POLA1 were also described. Somatic mutation are associated with more profound deficiency of POLA1, with develops into X-linked intellectual disability (XLID). In a case of non-XLPDR mutations, beside of type I interferon signature patients also display mild to medium signs of intellectual disability, cell cycle arrest, proportionate short stature, microcephaly and hypogonadism.[10]
Interactions
DNA dependent polymerase alpha (Pol α) has been shown to interact with MCM4 and GINS1,[8] Retinoblastoma protein,[11] PARP1[12][13] and RBMS1.[14]
References
- GRCh38: Ensembl release 89: ENSG00000101868 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000006678 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Entrez Gene: POLA1 polymerase (DNA directed), alpha 1".
- Madru C, Henneke G, Raia P, Hugonneau-Beaufet I, Pehau-Arnaudet G, England P, et al. (March 2020). "Structural basis for the increased processivity of D-family DNA polymerases in complex with PCNA". Nature Communications. 11 (1): 1591. Bibcode:2020NatCo..11.1591M. doi:10.1038/s41467-020-15392-9. PMC 7101311. PMID 32221299.
- Starokadomskyy P, Gemelli T, Rios JJ, Xing C, Wang RC, Li H, et al. (May 2016). "DNA polymerase-α regulates the activation of type I interferons through cytosolic RNA:DNA synthesis". Nature Immunology. 17 (5): 495–504. doi:10.1038/ni.3409. PMC 4836962. PMID 27019227.
- Starokadomskyy P, Wilton KM, Krzewski K, Lopez A, Sifuentes-Dominguez L, Overlee B, et al. (November 2019). "NK cell defects in X-linked pigmentary reticulate disorder". JCI Insight. 4 (21). doi:10.1172/jci.insight.125688. PMC 6948767. PMID 31672938.
- Starokadomskyy P, Sifuentes-Dominguez L, Gemelli T, Zinn AR, Dossi MT, Mellado C, et al. (November 2017). "Evolution of the skin manifestations of X-linked pigmentary reticulate disorder". The British Journal of Dermatology. 177 (5): e200–e201. doi:10.1111/bjd.15586. PMC 5640471. PMID 28407217.
- Van Esch H, Colnaghi R, Freson K, Starokadomskyy P, Zankl A, Backx L, et al. (May 2019). "Defective DNA Polymerase α-Primase Leads to X-Linked Intellectual Disability Associated with Severe Growth Retardation, Microcephaly, and Hypogonadism". American Journal of Human Genetics. 104 (5): 957–967. doi:10.1016/j.ajhg.2019.03.006. PMC 6506757. PMID 31006512.
- Takemura M, Kitagawa T, Izuta S, Wasa J, Takai A, Akiyama T, Yoshida S (November 1997). "Phosphorylated retinoblastoma protein stimulates DNA polymerase alpha". Oncogene. 15 (20): 2483–2492. doi:10.1038/sj.onc.1201431. PMID 9395244.
- Dantzer F, Nasheuer HP, Vonesch JL, de Murcia G, Ménissier-de Murcia J (April 1998). "Functional association of poly(ADP-ribose) polymerase with DNA polymerase alpha-primase complex: a link between DNA strand break detection and DNA replication". Nucleic Acids Research. 26 (8): 1891–1898. doi:10.1093/nar/26.8.1891. PMC 147507. PMID 9518481.
- Simbulan CM, Suzuki M, Izuta S, Sakurai T, Savoysky E, Kojima K, et al. (January 1993). "Poly(ADP-ribose) polymerase stimulates DNA polymerase alpha by physical association". The Journal of Biological Chemistry. 268 (1): 93–99. doi:10.1016/S0021-9258(18)54119-3. PMID 8416979.
- Niki T, Galli I, Ariga H, Iguchi-Ariga SM (June 2000). "MSSP, a protein binding to an origin of replication in the c-myc gene, interacts with a catalytic subunit of DNA polymerase alpha and stimulates its polymerase activity". FEBS Letters. 475 (3): 209–212. doi:10.1016/S0014-5793(00)01679-3. PMID 10869558. S2CID 31594271.
External links
- PDBe-KB provides an overview of all the structure information available in the PDB for Human DNA polymerase alpha catalytic subunit
Further reading
- Pollok S, Stoepel J, Bauerschmidt C, Kremmer E, Nasheuer HP (February 2003). "Regulation of eukaryotic DNA replication at the initiation step". Biochemical Society Transactions. 31 (Pt 1): 266–269. doi:10.1042/BST0310266. PMID 12546699.
- Fisher PA, Korn D (September 1977). "DNA polymerase-alpha. Purification and structural characterization of the near homogeneous enzyme from human KB cells". The Journal of Biological Chemistry. 252 (18): 6528–6535. doi:10.1016/S0021-9258(17)39990-8. PMID 893425.
- Dornreiter I, Erdile LF, Gilbert IU, von Winkler D, Kelly TJ, Fanning E (February 1992). "Interaction of DNA polymerase alpha-primase with cellular replication protein A and SV40 T antigen". The EMBO Journal. 11 (2): 769–776. doi:10.1002/j.1460-2075.1992.tb05110.x. PMC 556510. PMID 1311258.
- Coverley D, Kenny MK, Lane DP, Wood RD (August 1992). "A role for the human single-stranded DNA binding protein HSSB/RPA in an early stage of nucleotide excision repair". Nucleic Acids Research. 20 (15): 3873–3880. doi:10.1093/nar/20.15.3873. PMC 334061. PMID 1508673.
- Popanda O, Thielmann HW (January 1992). "The function of DNA polymerases in DNA repair synthesis of ultraviolet-irradiated human fibroblasts". Biochimica et Biophysica Acta (BBA) - Gene Structure and Expression. 1129 (2): 155–160. doi:10.1016/0167-4781(92)90480-N. PMID 1730053.
- Collins KL, Kelly TJ (April 1991). "Effects of T antigen and replication protein A on the initiation of DNA synthesis by DNA polymerase alpha-primase". Molecular and Cellular Biology. 11 (4): 2108–2115. doi:10.1128/mcb.11.4.2108. PMC 359898. PMID 1848671.
- Martelli AM, Cocco L, Manzoli FA (February 1991). "On the association of DNA polymerase alpha activity with the nuclear matrix in HeLa cells". Cell Biology International Reports. 15 (2): 131–140. doi:10.1016/0309-1651(91)90104-Q. PMID 1903085.
- Pearson BE, Nasheuer HP, Wang TS (April 1991). "Human DNA polymerase alpha gene: sequences controlling expression in cycling and serum-stimulated cells". Molecular and Cellular Biology. 11 (4): 2081–2095. doi:10.1128/mcb.11.4.2081. PMC 359896. PMID 2005899.
- Matsumoto T, Eki T, Hurwitz J (December 1990). "Studies on the initiation and elongation reactions in the simian virus 40 DNA replication system". Proceedings of the National Academy of Sciences of the United States of America. 87 (24): 9712–9716. Bibcode:1990PNAS...87.9712M. doi:10.1073/pnas.87.24.9712. PMC 55243. PMID 2175912.
- Hsi KL, Copeland WC, Wang TS (November 1990). "Human DNA polymerase alpha catalytic polypeptide binds ConA and RCA and contains a specific labile site in the N-terminus". Nucleic Acids Research. 18 (21): 6231–6237. doi:10.1093/nar/18.21.6231. PMC 332486. PMID 2243771.
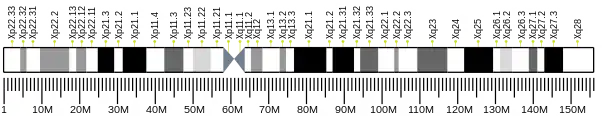
- Wang TS, Pearson BE, Suomalainen HA, Mohandas T, Shapiro LJ, Schröder J, Korn D (August 1985). "Assignment of the gene for human DNA polymerase alpha to the X chromosome". Proceedings of the National Academy of Sciences of the United States of America. 82 (16): 5270–5274. Bibcode:1985PNAS...82.5270W. doi:10.1073/pnas.82.16.5270. PMC 390549. PMID 2410918.
- Knorre DG, Lavrik OI, Nevinsky GA (May 1988). "Protein-nucleic acid interaction in reactions catalyzed with DNA polymerases". Biochimie. 70 (5): 655–661. doi:10.1016/0300-9084(88)90250-7. PMID 3139084.
- Nishida C, Reinhard P, Linn S (January 1988). "DNA repair synthesis in human fibroblasts requires DNA polymerase delta". The Journal of Biological Chemistry. 263 (1): 501–510. doi:10.1016/S0021-9258(19)57421-X. PMID 3335506.
- Wong SW, Wahl AF, Yuan PM, Arai N, Pearson BE, Arai K, et al. (January 1988). "Human DNA polymerase alpha gene expression is cell proliferation dependent and its primary structure is similar to both prokaryotic and eukaryotic replicative DNA polymerases". The EMBO Journal. 7 (1): 37–47. doi:10.1002/j.1460-2075.1988.tb02781.x. PMC 454213. PMID 3359994.
- Tsuda M, Masuyama M, Katsunuma T (December 1986). "Inhibition of human DNA polymerase alpha by alpha 1-antichymotrypsin". Cancer Research. 46 (12 Pt 1): 6139–6142. PMID 3490907.
- Jackson DA, Cook PR (November 1986). "Different populations of DNA polymerase alpha in HeLa cells". Journal of Molecular Biology. 192 (1): 77–86. doi:10.1016/0022-2836(86)90465-1. PMID 3820307.
- Miller MR, Seighman C, Ulrich RG (December 1985). "Inhibition of DNA replication and DNA polymerase alpha activity by monoclonal anti-(DNA polymerase alpha) immunoglobulin G and F(ab) fragments". Biochemistry. 24 (25): 7440–7445. doi:10.1021/bi00346a061. PMID 4084590.
- Bensch KG, Tanaka S, Hu SZ, Wang TS, Korn D (July 1982). "Intracellular localization of human DNA polymerase alpha with monoclonal antibodies". The Journal of Biological Chemistry. 257 (14): 8391–8396. doi:10.1016/S0021-9258(18)34344-8. PMID 7045121.
- Tanaka S, Hu SZ, Wang TS, Korn D (July 1982). "Preparation and preliminary characterization of monoclonal antibodies against human DNA polymerase alpha". The Journal of Biological Chemistry. 257 (14): 8386–8390. doi:10.1016/S0021-9258(18)34343-6. PMID 7085672.
- Eckert KA, Kunkel TA (November 1993). "Fidelity of DNA synthesis catalyzed by human DNA polymerase alpha and HIV-1 reverse transcriptase: effect of reaction pH". Nucleic Acids Research. 21 (22): 5212–5220. doi:10.1093/nar/21.22.5212. PMC 310639. PMID 7504813.