Amidophosphoribosyltransferase
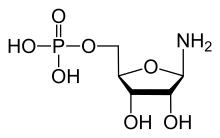
Amidophosphoribosyltransferase (ATase), also known as glutamine phosphoribosylpyrophosphate amidotransferase (GPAT), is an enzyme responsible for catalyzing the conversion of 5-phosphoribosyl-1-pyrophosphate (PRPP) into 5-phosphoribosyl-1-amine (PRA), using the amine group from a glutamine side-chain. This is the committing step in de novo purine synthesis. In humans it is encoded by the PPAT (phosphoribosyl pyrophosphate amidotransferase) gene.[5][6] ATase is a member of the purine/pyrimidine phosphoribosyltransferase family.
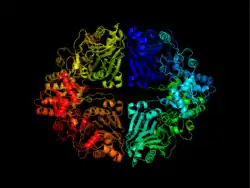
Structure and function
| amidophosphoribosyltransferase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| EC no. | 2.4.2.14 | ||||||||
| CAS no. | 9031-82-7 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / QuickGO | ||||||||
| |||||||||
The enzyme consists of two domains: a glutaminase domain that produces ammonia from glutamine by hydrolysis and a phosphoribosyltransferase domain that binds the ammonia to ribose-5-phosphate.[7] Coordination between the two active sites of enzyme give it special complexity.
The glutaminase domain is homologous to other N-terminal nucleophile (Ntn) hydrolases[7] such as carbamoyl phosphate synthetase (CPSase). Nine invariant residues among the sequences of all Ntn amidotransferases play key catalytic, substrate binding or structural roles. A terminal cysteine residue acts as the nucleophile in the first part of the reaction, analogous to the cysteine of a catalytic triad.[7][8] The free N terminus acts as a base to activate the nucleophile and protonate the leaving group in the hydrolytic reaction, in this case ammonia. Another key aspect of the catalytic site is an oxyanion hole which catalyzes the reaction intermediate, as shown in the mechanism below.[9]
The PRTase domain is homologous to many other PRTases involved in the purine nucleotide synthesis and salvage pathways. All PRTases involve the displacement of pyrophosphate in PRPP by a variety of nucleophiles.[10] ATase is the only PRTase that has ammonia as a nucleophile.[7] Pyrophosphate from PRPP is an excellent leaving group, so little chemical assistance is needed to promote catalysis. Rather, the primary function of the enzyme appears to be bringing the reactants together appropriately and preventing the wrong reaction, such as hydrolysis.[7]
Besides having their respective catalytic abilities, the two domains also coordinate with one another to ensure that all the ammonia produced from glutamine is transferred to PRPP and no other nucleophile than ammonia attacks PRPP. This is achieved mainly by blocking formation of ammonia until PRPP is bound and channelling the ammonia to the PRTase active site.[7]
Initial activation of the enzyme by PRPP is caused by a conformational change in a "glutamine loop", which repositions to be able to accept glutamine. This results in a 200-fold higher Km value for glutamine binding[11] Once glutamine has bound to the active site, further conformational changes bring the site into the enzyme, making it inaccessible.[7]
These conformational changes also result in the formation of a 20 Å long ammonia channel, one of the most striking features of this enzyme. This channel lacks any hydrogen bonding sites, to ensure easy diffusion of ammonia from one active site to the other. This channel ensures ammonia released from glutamine reaches the PRTase catalytic site, and it differs from the channel in CPSase[12] in that it is hydrophobic rather than polar, and transient rather than permanent.[7]
Reaction mechanism
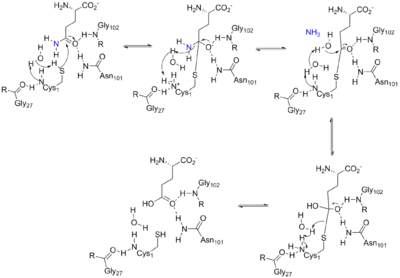
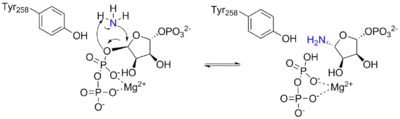
The overall reaction catalyzed by ATase is the following:
- PRPP + glutamine → PRA + glutamate + PPi
Within the enzyme, the reaction is broken down into two half-reactions that occur at different active sites:
- glutamine → NH
3 + glutamate - PRPP + NH
3 → PRA + PPi
The first part of the mechanism occurs in the active site of the glutaminase domain and releases an ammonia group from glutamine by hydrolysis. The ammonia released by the first reaction is then transferred to the active site of the phosphoribosyltransferase domain via a 20 Å channel, where it then binds to PRPP to form PRA.
Regulation
In an example of feedback inhibition, ATase is inhibited mainly by the end-products of the purine synthesis pathway, AMP, GMP, ADP, and GDP.[7] Each enzyme subunit from the homotetramer has two binding sites for these inhibitors. The allosteric (A) site overlaps with the site for the ribose-5-phosphate of PRPP, while the catalytic (C) site overlaps with the site for the pyrophosphate of PRPP.[7] The binding of specific nucleotide pairs to the two sites results in synergistic inhibition stronger than additive inhibition.[7][13][14] Inhibition occurs via a structural change in the enzyme where the flexible glutamine loop gets locked in an open position, preventing the binding of PRPP.[7]
Due to the chemical lability of PRA, which has a half-life of 38 seconds at pH 7.5 and 37 °C, researchers have suggested that the compound is channeled from Amidophosphoribosyltransferase to GAR synthetase in vivo.[15]
Interactive pathway map
Click on genes, proteins and metabolites below to link to respective articles.[§ 1]
- The interactive pathway map can be edited at WikiPathways: "FluoropyrimidineActivity_WP1601".
Gallery
References
- GRCh38: Ensembl release 89: ENSG00000128059 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000029246 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Entrez Gene: phosphoribosyl pyrophosphate amidotransferase".
- Brayton KA, Chen Z, Zhou G, Nagy PL, Gavalas A, Trent JM, Deaven LL, Dixon JE, Zalkin H (Feb 1994). "Two genes for de novo purine nucleotide synthesis on human chromosome 4 are closely linked and divergently transcribed". The Journal of Biological Chemistry. 269 (7): 5313–21. doi:10.1016/S0021-9258(17)37689-5. PMID 8106516.
- Smith JL (Dec 1998). "Glutamine PRPP amidotransferase: snapshots of an enzyme in action". Current Opinion in Structural Biology. 8 (6): 686–94. doi:10.1016/s0959-440x(98)80087-0. PMID 9914248.
- Smith JL, Zaluzec EJ, Wery JP, Niu L, Switzer RL, Zalkin H, Satow Y (Jun 1994). "Structure of the allosteric regulatory enzyme of purine biosynthesis". Science. 264 (5164): 1427–1433. Bibcode:1994Sci...264.1427S. doi:10.1126/science.8197456. PMID 8197456.
- "Overview for MACiE Entry M0214". EMBL-EBI. Archived from the original on 2015-04-02. Retrieved 2015-03-10.
- Musick WD (1981). "Structural features of the phosphoribosyltransferases and their relationship to the human deficiency disorders of purine and pyrimidine metabolism". CRC Critical Reviews in Biochemistry. 11 (1): 1–34. doi:10.3109/10409238109108698. PMID 7030616.
- Kim JH, Krahn JM, Tomchick DR, Smith JL, Zalkin H (Jun 1996). "Structure and function of the glutamine phosphoribosylpyrophosphate amidotransferase glutamine site and communication with the phosphoribosylpyrophosphate site". The Journal of Biological Chemistry. 271 (26): 15549–15557. doi:10.1074/jbc.271.26.15549. PMID 8663035.
- Thoden JB, Holden HM, Wesenberg G, Raushel FM, Rayment I (May 1997). "Structure of carbamoyl phosphate synthetase: a journey of 96 A from substrate to product". Biochemistry. 36 (21): 6305–6316. CiteSeerX 10.1.1.512.5333. doi:10.1021/bi970503q. PMID 9174345.
- Chen S, Tomchick DR, Wolle D, Hu P, Smith JL, Switzer RL, Zalkin H (Sep 1997). "Mechanism of the synergistic end-product regulation of Bacillus subtilis glutamine phosphoribosylpyrophosphate amidotransferase by nucleotides". Biochemistry. 36 (35): 10718–10726. doi:10.1021/bi9711893. PMID 9271502.
- Zhou G, Smith JL, Zalkin H (Mar 1994). "Binding of purine nucleotides to two regulatory sites results in synergistic feedback inhibition of glutamine 5-phosphoribosylpyrophosphate amidotransferase". The Journal of Biological Chemistry. 269 (9): 6784–6789. doi:10.1016/S0021-9258(17)37444-6. PMID 8120039.
- Antle VD, Liu D, McKellar BR, Caperelli CA, Hua M, Vince R (1996). "Substrate specificity of glycinamide ribonucleotide synthetase from chicken liver". The Journal of Biological Chemistry. 271 (14): 8192–5. doi:10.1074/jbc.271.14.8192. PMID 8626510.
Further reading
- Iwahana H, Oka J, Mizusawa N, Kudo E, Ii S, Yoshimoto K, Holmes EW, Itakura M (Jan 1993). "Molecular cloning of human amidophosphoribosyltransferase". Biochemical and Biophysical Research Communications. 190 (1): 192–200. doi:10.1006/bbrc.1993.1030. PMID 8380692.
- Gassmann MG, Stanzel A, Werner S (Nov 1999). "Growth factor-regulated expression of enzymes involved in nucleotide biosynthesis: a novel mechanism of growth factor action". Oncogene. 18 (48): 6667–76. doi:10.1038/sj.onc.1203120. PMID 10597272.
- Chen S, Nagy PL, Zalkin H (May 1997). "Role of NRF-1 in bidirectional transcription of the human GPAT-AIRC purine biosynthesis locus". Nucleic Acids Research. 25 (9): 1809–16. doi:10.1093/nar/25.9.1809. PMC 146651. PMID 9108165.
- Stanley W, Chu EH (1978). "Assignment of the gene for phosphoribosylpyrophosphate amidotransferase to the pter leads to q21 region of human chromosome 4". Cytogenetics and Cell Genetics. 22 (1–6): 228–31. doi:10.1159/000130943. PMID 752480.
- Maruyama K, Sugano S (Jan 1994). "Oligo-capping: a simple method to replace the cap structure of eukaryotic mRNAs with oligoribonucleotides". Gene. 138 (1–2): 171–4. doi:10.1016/0378-1119(94)90802-8. PMID 8125298.
- Bera AK, Chen S, Smith JL, Zalkin H (Dec 1999). "Interdomain signaling in glutamine phosphoribosylpyrophosphate amidotransferase". The Journal of Biological Chemistry. 274 (51): 36498–504. doi:10.1074/jbc.274.51.36498. PMID 10593947.
- Zalkin H, Dixon JE (1992). De novo purine nucleotide biosynthesis. pp. 259–87. doi:10.1016/s0079-6603(08)60578-4. ISBN 9780125400428. PMID 1574589.
{{cite book}}:|journal=ignored (help) - Suzuki Y, Yoshitomo-Nakagawa K, Maruyama K, Suyama A, Sugano S (Oct 1997). "Construction and characterization of a full length-enriched and a 5'-end-enriched cDNA library". Gene. 200 (1–2): 149–56. doi:10.1016/S0378-1119(97)00411-3. PMID 9373149.
External links
- Amidophosphoribosyl transferase at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- Human PPAT genome location and PPAT gene details page in the UCSC Genome Browser.
This article incorporates text from the United States National Library of Medicine, which is in the public domain.