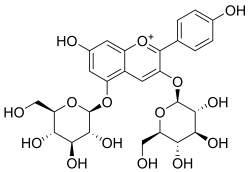
Pelargonin
Pelargonin is an anthocyanin. It is the 3,5-O-diglucoside of pelargonidin.
 | |
| Names | |
|---|---|
| IUPAC name
7-Hydroxy-2-(4-hydroxyphenyl)-3,5-bis{[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}-1λ4-benzopyran-1-ylium | |
| Other names
Pelargonidin 3,5-di-β-D-glucoside | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.037.584 |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C27H31O15+ | |
| Molar mass | 595.53 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Natural occurrences
Pelargonin is a pigment, found in barberries,[1] the petals of the scarlet pelargonium flower[2] pomegranates,[3] and red wine.[4]
See also
References
- Duke, James (1992). "Berberis vulgaris (Berberidaceae), United States Department of Agriculture, National Agricultural Library: Dr. Duke's Phytochemical and Ethnobotanical Databases". phytochem.nal.usda.gov.
- Saunders Currey, Geoffrey (1922). "The Colouring Matter of the Scarlet Pelargonium". J. Chem. Soc., Trans. 121: 319–323. doi:10.1039/CT9222100319.
- Gil, M.I.; Cherif, J.; Ayed, N.; Artes, F.; Tomasbarberan, F.A. (1995). "Influence of Cultivar, Maturity Stage and Geographical Location on the Juice Pigmentation of Tunisian Pomegranates". Zeitschrift für Lebensmittel-Untersuchung und Forschung. 201 (4): 361–364. doi:10.1007/BF01192733. S2CID 95566626.
- He, Fei; Liang, Na-Na; Mu, Lin; Pan, Qiu-Hong; Wang, Jun; Reeves, Michael J.; Duan, Chang-Qing (February 2012). "Anthocyanins and Their Variation in Red Wines I. Monomeric Anthocyanins and Their Color Expression". Molecules. 17 (2): 1571–1601. CiteSeerX 10.1.1.399.2775. doi:10.3390/molecules17021571. PMC 6268338. PMID 22314380.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.