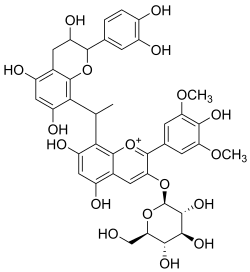
Malvidin glucoside-ethyl-catechin
Malvidin glucoside-ethyl-catechin is a flavanol-anthocyanin adduct.[1][2] Flavanol-anthocyanin adducts are formed during wine ageing through reactions between anthocyanins and tannins present in grape, with yeast metabolites such as acetaldehyde. Acetaldehyde-induced reactions yield ethyl-linked species such as malvidin glucoside-ethyl-catechin.[3][4]
 | |
| Names | |
|---|---|
| Preferred IUPAC name
8-{(1Ξ)-1-[(2R*,3S*)-2-(3,4-Dihydroxyphenyl)-3,5,7-trihydroxy-3,4-dihydro-2H-1-benzopyran-8-yl]ethyl}-5,7-dihydroxy-2-(4-hydroxy-3,5-dimethoxyphenyl)-3-{[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}-1λ4-benzopyran-1-ylium | |
| Other names
8,8-linked malvidin-3-glucose-ethyl-(epi)catechin | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| |
| |
| Properties | |
| C40H40O18 | |
| Molar mass | 809.75 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
This compound has a better color stability at pH 5.5 than malvidin-3O-glucoside. When the pH was increased from 2.2 to 5.5, the solution of the pigment became progressively more violet (λmax = 560 nm at pH 5.5), whereas similar solutions of the anthocyanin were almost colorless at pH 4.0.[5]
Other types of aldehyde, such as isovaleraldehyde, benzaldehyde, propionaldehyde, isobutyraldehyde, formaldehyde or 2-methylbutyraldehyde, show the same reactivity in model solutions.[6]
References
- Malvidin glucoside-ethyl-catechin on Yeast Metabolome Database
- Atanasova, Vessela; Fulcrand, Hélène; Cheynier, Véronique; Moutounet, Michel (2002). "Effect of oxygenation on polyphenol changes occurring in the course of wine-making". Analytica Chimica Acta. 458: 15–27. doi:10.1016/S0003-2670(01)01617-8.
- Morata, A; González, C; Suárez-Lepe, JA (2007). "Formation of vinylphenolic pyranoanthocyanins by selected yeasts fermenting red grape musts supplemented with hydroxycinnamic acids". International Journal of Food Microbiology. 116 (1): 144–52. doi:10.1016/j.ijfoodmicro.2006.12.032. PMID 17303275.
- Asenstorfer, Robert E.; Lee, David F.; Jones, Graham P. (2006). "Influence of structure on the ionisation constants of anthocyanin and anthocyanin-like wine pigments". Analytica Chimica Acta. 563 (1–2): 10–14. doi:10.1016/j.aca.2005.09.040.
- Escribano-Bailón, Teresa; Álvarez-García, Marta; Rivas-Gonzalo, Julian C.; Heredia, Francisco J.; Santos-Buelga, Celestino (2001). "Color and Stability of Pigments Derived from the Acetaldehyde-Mediated Condensation between Malvidin 3-O-Glucoside and (+)-Catechin". Journal of Agricultural and Food Chemistry. 49 (3): 1213–7. doi:10.1021/jf001081l. PMID 11312838.
- Pissarra, J.; Mateus, N.; Rivas-Gonzalo, J.; Santos Buelga, C.; Freitas, V. (2003). "Reaction Between Malvidin 3-Glucoside and (+)-Catechin in Model Solutions Containing Different Aldehydes". Journal of Food Science. 68 (2): 476–481. doi:10.1111/j.1365-2621.2003.tb05697.x. INIST:15183380.