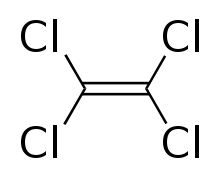
Tetrachloroethylene
| |||
 | |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Tetrachloroethene | |||
| Other names | |||
| Identifiers | |||
3D model (JSmol) |
|||
| 1304635 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.004.388 | ||
| EC Number |
| ||
| 101142 | |||
| KEGG | |||
PubChem CID |
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 1897 | ||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C2Cl4 | |||
| Molar mass | 165.82 g/mol | ||
| Appearance | Clear, very refractive, colorless liquid | ||
| Odor | Mild minty and sweetish, chloroform-like[3] | ||
| Density | 1.622 g/cm3 | ||
| Melting point | −22.0 to −22.7 °C (−7.6 to −8.9 °F; 251.2 to 250.5 K) | ||
| Boiling point | 121.1 °C (250.0 °F; 394.2 K) | ||
| 0.15 g/L (25 °C) | |||
| Vapor pressure | 14 mmHg (20 °C)[3] | ||
| −81.6·10−6 cm3/mol | |||
Refractive index (nD) |
1.505 | ||
| Viscosity | 0.89 cP at 25 °C | ||
| Hazards | |||
| GHS labelling: | |||
  | |||
| Warning | |||
| H351, H411 | |||
| P201, P202, P273, P281, P308+P313, P391, P405, P501 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | Not flammable | ||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose) |
3420 mg/kg (oral, rat)[5] 2629 mg/kg (oral, rat), >10000 mg/kg (dermal, rat)[6] | ||
LC50 (median concentration) |
4000 ppm (rat, 4 hr) 5200 ppm (mouse, 4 hr) 4964 ppm (rat, 8 hr)[7] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible) |
TWA 100 ppm C 200 ppm (for 5 minutes in any 3-hour period), with a maximum peak of 300 ppm[3] | ||
REL (Recommended) |
Ca Minimize workplace exposure concentrations.[3] | ||
IDLH (Immediate danger) |
Ca [150 ppm][3] | ||
| Safety data sheet (SDS) | External MSDS | ||
| Related compounds | |||
Related organohalides |
Tetrafluoroethylene Tetrabromoethylene Tetraiodoethylene | ||
Related compounds |
Trichloroethylene Dichloroethylene Tetrachloroethane Carbon tetrachloride | ||
| Supplementary data page | |||
| Tetrachloroethylene (data page) | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |||
Tetrachloroethylene, also known under the systematic name tetrachloroethene, or perchloroethylene, and abbreviations such as "perc" (or "PERC"), and "PCE", is a chlorocarbon with the formula Cl2C=CCl2. It is a colorless liquid widely used for dry cleaning of fabrics, hence it is sometimes called "dry-cleaning fluid". It also has its uses as an effective automotive brake cleaner. It has a mild sweet odor, similar to the smell of chloroform, detectable by most people at a concentration of 1 part per million (1 ppm).
History and production
French chemist Henri Victor Regnault first synthesized tetrachloroethylene in 1839 by thermal decomposition of hexachloroethane following Michael Faraday's 1820 synthesis of protochloride of carbon (carbon tetrachloride).
- C2Cl6 → C2Cl4 + Cl2
Faraday was previously falsely credited for the synthesis of tetrachloroethylene, which in reality, was carbon tetrachloride. While trying to make Faraday's "protochloride of carbon", Regnault found that his compound was different from Faraday's. Victor Regnault stated "According to Faraday, the chloride of carbon boiled around 70 °C (158 °F) to 77 °C (171 °F) degrees Celsius but mine did not begin to boil until 120 °C (248 °F) ".[8]
Few years after its discovery, in the 1840s, Tetrachloroethylene was named Chlorethose by Auguste Laurent. The -ose ending was explained as the fourfold replacement of the hydrogens in ethylene. If only one atom of hydrogen was replaced, the word would end with -ase. By Laurent's logic, vinyl chloride would be named Chlorase.[9]
Tetrachloroethylene can be made by passing chloroform vapour through a red-hot tube, the side products include hexachlorobenzene and hexachloroethane, as reported in 1886.[10]
Most tetrachloroethylene is produced by high-temperature chlorinolysis of light hydrocarbons. The method is related to Faraday's method since hexachloroethane is generated and thermally decomposes.[11] Side products include carbon tetrachloride, hydrogen chloride, and hexachlorobutadiene.
Several other methods have been developed. When 1,2-dichloroethane is heated to 400 °C with chlorine, tetrachloroethylene is produced by the chemical reaction:
- ClCH2CH2Cl + 3 Cl2 → Cl2C=CCl2 + 4 HCl
This reaction can be catalyzed by a mixture of potassium chloride and aluminium chloride or by activated carbon. Trichloroethylene is a major byproduct, which is separated by distillation.
Worldwide production was about 1 million metric tons (980,000 long tons; 1,100,000 short tons) in 1985.[11]
Although in very small amounts, tetrachloroethylene occurs naturally in volcanoes along with trichloroethylene.[12]
Uses

Tetrachloroethylene is an excellent nonpolar solvent for organic materials. Otherwise, it is volatile, highly stable and nonflammable, and has low toxicity. For these reasons, it has been widely used in dry cleaning worldwide since the 1930s. The chemist Sylvia Stoesser (1901–1991) suggested Tetrachloroethylene to be used in dry cleaning as an alternative to highly flammable dry cleaning solvents such as naphtha.[13]
It is also used to degrease metal parts in the automotive and other metalworking industries, usually as a mixture with other chlorocarbons. It appears in a few consumer products including paint strippers, aerosol preparations and spot removers.
Historical applications
Tetrachloroethylene was once extensively used as an intermediate in the manufacture of HFC-134a and related refrigerants. In the early 20th century, tetrachloroethene was used for the treatment of hookworm infestation.[14][15]
Health and safety
The acute toxicity of tetrachloroethylene is moderate to low. Reports of human injury are uncommon despite its wide usage in dry cleaning and degreasing.[16]
Despite the advantages of tetrachloroethylene, many have called for its replacement from widespread commercial use. It has been described as a possible "neurotoxicant, liver and kidney toxicant and reproductive and developmental toxicant ... a 'potential occupational carcinogen'"..[17]
Testing for exposure
Tetrachloroethylene exposure can be evaluated by a breath test, analogous to breath-alcohol measurements. Also, for acute exposures, tetrachloroethylene in expired air can be measured.[18] Tetrachloroethylene can be detected in the breath for weeks following a heavy exposure. Tetrachloroethylene and its metabolite trichloroacetic acid, can be detected in the blood.
In Europe, the Scientific Committee on Occupational Exposure Limits (SCOEL) recommends for tetrachloroethylene an occupational exposure limit (8-hour time-weighted average) of 20 ppm and a short-term exposure limit (15 min) of 40 ppm.[19]
Remediation and degradation
In principle, tetrachloroethylene contamination can be remediated by chemical treatment. Chemical treatment involves reducing metals such as iron powder.
Bioremediation usually entails reductive dechlorination under anaerobic conditions by Dehalococcoides spp.[20] Under aerobic conditions, degradation may occur via cometabolism by Pseudomonas sp.[21] Products of biological reductive dechlorination include trichloroethene, cis-1,2-dichloroethene, vinyl chloride, ethene and chloride.
References
- C. Chabrie "General Method for the Preparation of Carbon Fluorides" in Journal - Chemical Society, London. (1890). UK: Chemical Society.
- Justus Liebigs Annalen der Chemie. (1845). Germany: Verlag Chemie. Page 277
- NIOSH Pocket Guide to Chemical Hazards. "#0599". National Institute for Occupational Safety and Health (NIOSH).
- "Compound Summary: Tetrachloroethylene". PubChem. Retrieved 9 September 2020.
- Sigma Aldrich Tetrachloroethylene MSDS
- Fischer Scientific Tetrachloroethylene MSDS
- "Tetrachloroethylene". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- V. Regnault (1839) "Sur les chlorures de carbone CCl et CCl2" (On the chlorides of carbon CCl and CCl2 ), Annales de Chimie et de Physique, vol. 70, pages 104-107. Reprinted in German as: V. Regnault (1839). "Ueber die Chlorverbindungen des Kohlenstoffs, C2Cl2 und CCl2". Annalen der Pharmacie. 30 (3): 350–352. doi:10.1002/jlac.18390300310.
- Transactions of the Pharmaceutical Meetings. (1847). UK: J. Churchill. page 548
- W. Ramsay and S. Young, Jahresberichte, 1886, p. 628
- M. Rossberg et al. "Chlorinated Hydrocarbons" in Ullmann's Encyclopedia of Industrial Chemistry, 2006, Wiley-VCH, Weinheim. doi:10.1002/14356007.a06_233.pub2
- Gribble, G. W. (1996). "Naturally occurring organohalogen compounds – A comprehensive survey". Progress in the Chemistry of Organic Natural Products. 68 (10): 1–423. doi:10.1021/np50088a001. PMID 8795309.
- Amos, J. Lawrence (1990). "Chlorinated solvents". In Boundy, Ray H.; Amos, J. Lawrence (eds.). A History of the Dow Chemical Physics Lab : the freedom to be creative. New York and Basel: Marcel Dekker, Inc. pp. 71–79.
- Young, M.D.; et al. (1960). "The Comparative Efficacy of Bephenium Hydroxynaphthoate and Tetrachloroethylene against Hookworm and other Parasites of Man". American Journal of Tropical Medicine and Hygiene. 9 (5): 488–491. doi:10.4269/ajtmh.1960.9.488. PMID 13787477.
- "Clinical Aspects and Treatment of the More Common Intestinal Parasites of Man (TB-33)". Veterans Administration Technical Bulletin 1946 & 1947. 10: 1–14. 1948.
- E.-L. Dreher; T. R. Torkelson; K. K. Beutel (2011). "Chlorethanes and Chloroethylenes". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.o06_o01. ISBN 978-3527306732.
- Ceballos, Diana M.; Fellows, Katie M.; Evans, Ashley E.; Janulewicz, Patricia A.; Lee, Eun Gyung; Whittaker, Stephen G. (2021). "Perchloroethylene and Dry Cleaning: It's Time to Move the Industry to Safer Alternatives". Frontiers in Public Health. 9: 638082. doi:10.3389/fpubh.2021.638082. PMC 7973082. PMID 33748070.
- "Tetrachloroethylene Toxicity: Section 3.1. Evaluation and Diagnosis | Environmental Medicine | ATSDR". www.atsdr.cdc.gov. 9 February 2021. Retrieved 2 March 2023.
- "SCOEL recommendations". 22 April 2011. Retrieved 22 April 2011.
- Ghattas, Ann-Kathrin; Fischer, Ferdinand; Wick, Arne; Ternes, Thomas A. (2017). "Anaerobic biodegradation of (Emerging) organic contaminants in the aquatic environment". Water Research. 116: 268–295. doi:10.1016/j.watres.2017.02.001. PMID 28347952.
- Ryoo, D.; Shim, H.; Arenghi, F. L. G.; Barbieri, P.; Wood, T. K. (2001). "Tetrachloroethylene, Trichloroethylene, and Chlorinated Phenols Induce Toluene-o-xylene Monooxoygenase Activity in Pseudomonas stutzeri OX1". Appl Microbiol Biotechnol. 56 (3–4): 545–549. doi:10.1007/s002530100675. PMID 11549035. S2CID 23770815.
Further reading
- "Toxicological Profile for Tetrachloroethene". Agency for Toxic Substances and Disease Registry. 1997.
- Doherty, R.E. (2000). "A History of the Production and Use of Carbon Tetrachloride, Tetrachloroethylene, Trichloroethylene and 1,1,1-Trichloroethane in the United States: Part 1 - Historical Background; Carbon Tetrachloride and Tetrachloroethylene". Environmental Forensics. 1 (2): 69–81. doi:10.1006/enfo.2000.0010. S2CID 97680726.
External links
- ATSDR Case Studies in Environmental Medicine: Tetrachloroethylene Toxicity U.S. Department of Health and Human Services
- Tetrachloroethylene (Perchloroethylene) U.S. Department of Health and Human Services
- Australian National Pollutant Inventory (NPI) page
- Sustainable uses and Industry recommendations