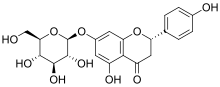
Prunin
Prunin is a flavanone glycoside found in immature citrus fruits[1] and in tomatoes.[3] Its aglycone form is called naringenin.
 | |
 | |
| Names | |
|---|---|
| IUPAC name
(2S)-7-(β-D-Glucopyranosyloxy)-4′,5-dihydroxyflavan-4-one | |
| Systematic IUPAC name
(2S)-5-Hydroxy-2-(4-hydroxyphenyl)-7-{[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}-2,3-dihydro-4H-1-benzopyran-4-one | |
| Other names
Naringenin-7-O-glucoside | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.007.696 |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C21H22O10 | |
| Molar mass | 434.397 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Metabolism
Glucosidase breaks prunin into glucose and naringenin.
References
- Berhow, Mark A.; Vandercook, Carl E. (1989). "Biosynthesis of naringin and prunin in detached grapefruit". Phytochemistry. 28 (6): 1627–1630. doi:10.1016/S0031-9422(00)97813-0. ISSN 0031-9422.
- Improved characterization of tomato polyphenols using liquid chromatography/electrospray ionization linear ion trap quadrupole Orbitrap mass spectrometry and liquid hromatography/electrospray ionization tandem mass spectrometry. Anna Vallverdu´-Queralt, Olga Jauregui, Alexander Medina-Remon, Cristina Andres-Lacueva and Rosa M. Lamuela-Raventos, Rapid Commun. Mass Spectrom., 2010, volume 24, pages 2986–2992, doi:10.1002/rcm.4731
Bibliography
- Habelt, Konrad; Pittner, Fritz (1983). "A rapid method for the determination of naringin, prunin, and naringenin applied to the assay of naringinase". Analytical Biochemistry. 134 (2): 393–397. doi:10.1016/0003-2697(83)90314-7. ISSN 0003-2697. PMID 6418025.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.