Sakuranetin
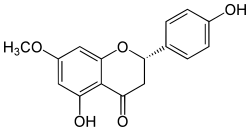
Sakuranetin is a flavan-on, the 7-methoxy derivative of naringenin, found in Polymnia fruticosa[1] and rice, where it acts as a phytoalexin against spore germination of Pyricularia oryzae.[2]
 | |
| Names | |
|---|---|
| IUPAC name
(2S)-4′,5-Dihydroxy-7-methoxyflavan-4-one | |
| Systematic IUPAC name
(2S)-5-Hydroxy-2-(4-hydroxyphenyl)-7-methoxy-2,3-dihydro-4H-1-benzopyran-4-one | |
| Other names
Naringenin 7-methyl ether | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.019.073 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C16H14O5 | |
| Molar mass | 286.27 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Metabolism
- biosynthesis
Naringenin 7-O-methyltransferase uses naringenin to yield sakuranetin, with S-adenosyl-methionine as the methyl donor.[3]
- biodegradation
In compounds like 7-methoxylated flavanones like sakuranetin, demethylation followed by sulfation occur in model organism Cunninghamella elegans.[4]
References
- Sakuranetin on home.ncifcrf.gov
- Sakuranetin, a flavonone phytoalexin from ultraviolet-irradiated rice leaves, Kodama O., Miyakawa J., Akatsuka T., Kiyosawa S, 1992
- A Methyltransferase for Synthesis of the Flavanone Phytoalexin Sakuranetin in Rice Leaves, Randeep Rakwala, Morifumi Hasegawab and Osamu Kodama, 1996
- Ibrahim, A. R.; Galal, A. M.; Ahmed, M. S.; Mossa, G. S. (2003). "O-demethylation and sulfation of 7-methoxylated flavanones by Cunninghamella elegans". Chemical & Pharmaceutical Bulletin. 51 (2): 203–206. doi:10.1248/cpb.51.203. PMID 12576658. INIST:14569933.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.