Root microbiome
The root microbiome (also called rhizosphere microbiome) is the dynamic community of microorganisms associated with plant roots.[1] Because they are rich in a variety of carbon compounds, plant roots provide unique environments for a diverse assemblage of soil microorganisms, including bacteria, fungi and archaea. The microbial communities inside the root and in the rhizosphere are distinct from each other,[2] and from the microbial communities of bulk soil,[3] although there is some overlap in species composition.
| Part of a series on |
| Microbiomes |
|---|
 |
|
Different microorganisms, both beneficial and harmful affect development and physiology of plants. Beneficial microorganisms include bacteria that fix nitrogen, promote plant growth, mycorrhizal fungi, mycoparasitic fungi, protozoa and certain biocontrol microorganisms.[1] Pathogenic microorganisms also span certain bacteria, pathogenic fungi and certain nematodes that can colonize the rhizosphere. Pathogens are able to compete with protective microbes and break through innate plant defense mechanisms.[1] Apart from microbes that cause plant diseases, certain bacteria that are pathogenic and can be carried over to humans, such as Salmonella, enterohaemorhagic Escherichia coli, Burkholedria (ceno)cepacia, Pseudomonas aeruginosa, and Stenotrophomonas maltophilia can also be detected in root associated microbiome and in plant tissues.[1]
Root microbiota affect plant host fitness and productivity in a variety of ways. Members of the root microbiome benefit from plant sugars or other carbon rich molecules. Individual members of the root microbiome may behave differently in association with different plant hosts,[4] or may change the nature of their interaction (along the mutualist-parasite continuum) within a single host as environmental conditions or host health change.[5]
Despite the potential importance of the root microbiome for plants and ecosystems, our understanding of how root microbial communities are assembled is in its infancy.[6][7] This is in part because until recent advances in sequencing technologies, root microbes were difficult to study due to high species diversity, the large number of cryptic species, and the fact that most species have yet to be retrieved in culture.[8] Evidence suggests both biotic (such as host identity and plant neighbor) and abiotic (such as soil structure and nutrient availability) factors affect community composition.[9][10][11][12][13]
Function
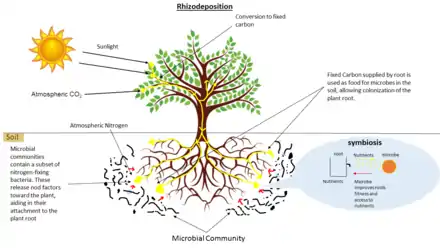
Types of symbioses
Root associated microbes include fungi, bacteria, and archaea. In addition, other organisms such as viruses, algae, protozoa, nematodes and arthropods are part of root microbiota.[1] Symbionts associated with plant roots subsist off of photosynthetic products (carbon rich molecules) from the plant host and can exist anywhere on the mutualist/parasite continuum.
Root symbionts may improve their host's access to nutrients,[14][15][16] produce plant-growth regulators,[17] improve environmental stress tolerance of their host,[18][19][20] induce host defenses and systemic resistance against pests or pathogens,[21][22][23] or be pathogenic.[24] Parasites consume carbon from the plant without providing any benefit, or providing too little benefit relative to what they cost in carbon, thereby compromising host fitness. Symbionts may be biotrophic (subsisting off of living tissue) or necrotrophic (subsisting off of dead tissue).
Mutualist-parasite continuum
While some microbes may be purely mutualistic or parasitic, many may behave one way or the other depending on the host species with which it is associated, environmental conditions, and host health.[4] A host's immune response controls symbiont infection and growth rates.[4] If a host's immune response is not able to control a particular microbial species, or if host immunity is compromised, the microbe-plant relationship will likely reside somewhere nearer the parasitic side of the mutualist-parasite continuum. Similarly, high nutrients can push some microbes into parasitic behavior, encouraging unchecked growth at a time when symbionts are no longer needed to aid with nutrient acquisition.[4]
Composition
Roots are colonized by fungi, bacteria and archaea. Because they are multicellular, fungi can extend hyphae from nutrient exchange organs within host cells into the surrounding rhizosphere and bulk soil. Fungi that extend beyond the root surface and engage in nutrient-carbon exchange with the plant host are commonly considered to be mycorrhizal, but external hyphae can also include other endophytic fungi. Mycorrhizal fungi can extend a great distance into bulk soil,[5] thereby increasing the root system's reach and surface area, enabling mycorrhizal fungi to acquire a large percentage of its host plant's nutrients. In some ecosystems, up to 80% of plant nitrogen and 90% of plant phosphorus is acquired by mycorrhizal fungi.[14] In return, plants may allocate ~20–40% of their carbon to mycorrhizae.[25]
Mycorrhizae
Mycorrhizal (from greek) literally means "fungus roots" and defines symbiotic interaction between plants and fungus. Fungi are important to decompose and recycle organic material; however, the boundaries between pathogenic and symbiotic lifestyles of fungi are not always clear-cut. Most of the time the association is symbiotic with fungus improving acquisition of nutrients and water from soil or increasing stress tolerance and fungus benefiting from carbohydrates produced by plant.[26] Mycorrhizae include a broad variety of root-fungi interactions characterized by mode of colonization. Essentially all plants form mycorrhizal associations, and there is evidence that some mycorrhizae transport carbon and other nutrients not just from soil to plant, but also between different plants in a landscape.[5] The main groups include ectomycorrhizae, arbuscular mycorhizae, ericoid mycorrhizae, orchid mycorrhizae, and monotropoid mycorrhizae. Monotropoid mycorrhizae are associated with plants in the monotropaceae, which lack chlorophyll. Many Orchids are also achlorophyllous for at least part of their life cycle. Thus these mycorrhizal-plant relationships are unique because the fungus provides the host with carbon as well as other nutrients, often by parasitizing other plants.[5] Achlorophyllous plants forming these types of mycorrhizal associations are called mycoheterotrophs.
Endophytes
Endophytes grow inside the plant tissues—roots, stems, leaves—mostly symptomless; however, when plant ages they can become slightly pathogenic.[26] They may colonize inter-cellular spaces, the root cells themselves, or both. Rhizobia and dark septate endophytes (which produce melanin, an antioxidant that may provide resilience against a variety of environmental stresses[27]) are famous examples.
Bacteria
Zone of soil surrounding the roots is rich in nutrients released by plants and is therefore attractive growth medium for both beneficial and pathogenic bacteria. Root associated beneficial bacteria promote plant growth and provide protection from pathogens. They are mostly rhizobacteria that belong to Pseudomonadota and Bacillota, with many examples from Pseudomonas and Bacillus genera.[1] Rhizobium species colonize legume roots forming nodule structures. In response to root exudates, rhizobia produce Nod signalling factors that are recognized by legumes and induce formation of nodules on plant roots.[28] Within these structures Rhizobium perform nitrogen fixation that proves plant with nitrogen source. In turn, plants provide bacteria with carbon source to energize the nitrogen fixation.[29][30] In addition to nitrogen fixation, Azospirillum species promote plant growth through production of growth phytohormones (auxins, cytokinins, gibberellins). Due to these phytohormones root hairs expand to occupy larger area and better acquire water and nutrients.[29][31] Pathogenic bacteria that infect plants infect plant roots are most commonly from Pectobacterium, Ralstonia, Dickeya and Agrobacterium genera. Among the most notorious are Pectobacterium carotovorum, Pectobacterium atrosepticum, Ralstonia solanacearum, Dickeya dadanthi, Dickeya solani and Agrobacterium tumefaciens.
Bacteria attach to roots in a certain biphasic mechanism with two steps—first weak, non-specific binding and then switch to second strong irreversible residence phase. Both beneficial and pathogenic bacteria attach in this fashion. Bacteria can stay attached to the outer surface or, certain endophytes or pathogens can colonize the inner root.[29] Primary attachment is governed by chemical forces or certain extracellular structures such as pili or flagella. Secondary attachment is mainly characterized by synthesis of cellulose, extracellular fibrils and specific attachment factors such as surface proteins that help bacteria aggregate and form colonies.[29]
Archaea
Though archaea are traditionally thought of as extremophiles, microbes belonging to extreme environments, advances in metagenomics and gene sequencing reveal that archaea are ubiquitous, found in nearly any environment including the root microbiome.[8][32][33][34][35][36] For example, root-colonizing archaea have been discovered in maize,[33] rice,[37] wheat,[34] and mangroves.[38] Methanogen and ammonium-oxidizing archaea are prevalent members of the root microbiome, especially in anaerobic soils and wetlands.[32][39][40][41] Archaeal phyla found in the root microbiome include Euryarchaeota,[32][40][42] Nitrososphaerota (formerly Thaumarchaeota),[32][42] and Thermoproteota (formerly Crenarchaeota).[40]
The presence and relative abundance of archaea in various environments suggest that they likely play an important role in the root microbiome.[32] Archaea have been found to promote plant growth and development, provide stress tolerance, improve nutrient uptake and protect against pathogens.[32][36][43] For example, Arabidopsis thaliana colonized with an ammonia-oxidizing soil archaea, Nitrosocosmicus oleophilius, exhibited increased shoot weight, photosynthetic activity and immune response.[43]
Examination of microbial communities in soil and roots identify archaeal organisms and genes that occupy functions similar to that of to bacteria and fungi, such as auxin synthesis, protection against abiotic stress and nitrogen fixation.[36][44] In some cases, key genes for plant growth and development, such as metabolism and cell wall synthesis, are more prevalent in archaea than bacteria.[36]
Archaeal presence in the root microbiome can also be affected by plant hosts, which can change the diversity, presence and health of archaeal communities.[8][38][45]
Viruses
Viruses also infect plants via the roots; however, to penetrate the root tissues they typically use vectors such as nematodes or fungi.[1]
Assembly mechanisms
There is an ongoing debate regarding what mechanisms are responsible for assembling individual microbes into communities. There are two primary competing hypotheses. One is that "everything is everywhere, but the environment selects," meaning biotic and abiotic factors pose the only constraints, through natural selection, to which microbes colonize what environments. This is called the niche hypothesis, and its counterpart is the hypothesis that neutral processes, such as distance and geographic barriers to dispersal, control microbial community assembly when taxa are equally fit within an environment. In this hypothesis, differences between individual taxa in modes and reach of dispersal explain the differences in microbial communities of different environments.[7] Most likely, both natural selection and neutral processes affect microbial community assembly, though certain microbial taxa may be more restricted by one process or the other depending on their physiological restrictions and mode of dispersion.[7]
Microbial dispersal mechanisms include wind, water, and hitchhiking on more mobile macrobes. Microbial dispersion is difficult to study, and little is known about its effect on microbial community assembly relative to the effect of abiotic and biotic assembly mechanisms,[7] particularly in roots. For this reason only assembly mechanisms that fit within the niche hypothesis are discussed below.
The taxa within root microbial communities seem to be drawn from the surrounding soil, though the relative abundance of various taxa may differ greatly from those found in bulk soil due to unique niches in the root and rhizosphere.[8]
Biotic assembly mechanisms
Different parts of the root are associated with different microbial communities. For example, fine roots, root tips, and the main root are all associated with different communities,[8][46] and the rhizosphere, root surface, and root tissue are all associated with different communities,[2][3] likely due to the unique chemistry and nutrient status of each of these regions. Additionally different plant species, and even different cultivars, harbor different microbial communities,[9][10][46] probably due to host specific immune responses[4] and differences in carbon root exudates.[47] Host age affects root microbial community composition, likely for similar reasons as host identity.[8] The identity of neighboring vegetation has also been shown to impact a host plant's root microbial community composition.[9][10][48][49]
Abiotic assembly mechanisms
Abiotic mechanisms also affect root microbial community assembly[9][10][11][12][13] because individual taxa have different optima along various environmental gradients, such as nutrient concentrations, pH, moisture, temperature, etc. In addition to chemical and climatic factors, soil structure and disturbance impact root biotic assembly.[8]
Succession
The root microbiome is dynamic, fluid within the constraints imposed by the biotic and abiotic environment. As in macroecological systems, the historical trajectory of the microbiotic community may partially determine the present and future community. Due to antagonistic and mutualistic interactions between microbial taxa, the taxa colonizing a root at any given moment could be expected to influence which new taxa are acquired, and therefore how the community responds to changes in the host or environment.[7] While the effect of initial community on microbial succession has been studied in various environmental samples, human microbiome, and laboratory settings, it has yet to be studied in roots.
See also
References
- Mendes R, Garbeva P, Raaijmakers JM (September 2013). "The rhizosphere microbiome: significance of plant beneficial, plant pathogenic, and human pathogenic microorganisms". FEMS Microbiology Reviews. 37 (5): 634–63. doi:10.1111/1574-6976.12028. PMID 23790204.
- Gottel NR, Castro HF, Kerley M, et al. (September 2011). "Distinct microbial communities within the endosphere and rhizosphere of Populus deltoides roots across contrasting soil types". Applied and Environmental Microbiology. 77 (17): 5934–44. Bibcode:2011ApEnM..77.5934G. doi:10.1128/AEM.05255-11. PMC 3165402. PMID 21764952.
- Nguyen C (2009), "Rhizodeposition of Organic C by Plant: Mechanisms and Controls", Sustainable Agriculture, pp. 97–123, doi:10.1007/978-90-481-2666-8_9, ISBN 978-90-481-2665-1
- Kogel KH, Franken P, Hückelhoven R (August 2006). "Endophyte or parasite—what decides?". Current Opinion in Plant Biology. 9 (4): 358–63. doi:10.1016/j.pbi.2006.05.001. PMID 16713330.
- Smith SE, Read DJ (2010). Mycorrhizal symbiosis (3rd ed.). New York, NY: Academic Press. ISBN 978-0-08-055934-6.
- Kristin A, Miranda H (2013). "The root microbiota—a fingerprint in the soil?". Plant and Soil. 370 (1–2): 671–86. doi:10.1007/s11104-013-1647-7. S2CID 14961515.
- Nemergut DR, Schmidt SK, Fukami T, O'Neill SP, Bilinski TM, Stanish LF, Knelman JE, Darcy JL, Lynch RC, Wickey P, Ferrenberg S (September 2013). "Patterns and processes of microbial community assembly". Microbiology and Molecular Biology Reviews. 77 (3): 342–56. doi:10.1128/MMBR.00051-12. PMC 3811611. PMID 24006468.
- Buée M, De Boer W, Martin F, van Overbeek L, Jurkevitch E (2009). "The rhizosphere zoo: An overview of plant-associated communities of microorganisms, including phages, bacteria, archaea, and fungi, and of some of their structuring factors". Plant and Soil. 321 (1–2): 189–212. doi:10.1007/s11104-009-9991-3. S2CID 45768977.
- Dean SL, Farrer EC, Taylor DL, Porras-Alfaro A, Suding KN, Sinsabaugh RL (March 2014). "Nitrogen deposition alters plant-fungal relationships: linking belowground dynamics to aboveground vegetation change". Molecular Ecology. 23 (6): 1364–78. doi:10.1111/mec.12541. PMID 24112704. S2CID 15540300.
- Dean SL, Farrer EC, Porras-Alfaro A, Suding KN, Sinsabaugh RL (2014). "Assembly of root-associated bacteria communities: interactions between abiotic and biotic factors". Environmental Microbiology Reports. 7 (1): 102–110. doi:10.1111/1758-2229.12194. PMID 25870878.
- Hardoim PR, Andreote FD, Reinhold-Hurek B, Sessitsch A, van Overbeek LS, van Elsas JD (July 2011). "Rice root-associated bacteria: insights into community structures across 10 cultivars". FEMS Microbiology Ecology. 77 (1): 154–64. doi:10.1111/j.1574-6941.2011.01092.x. PMC 4339037. PMID 21426364.
- Egerton-Warburton, Louise M.; Johnson, Nancy Collins; Allen, Edith B. (November 2007). "Mycorrhizal Community Dynamics following Nitrogen Fertilization: A Cross-Site Test in Five Grasslands". Ecological Monographs. 77 (4): 527–44. doi:10.1890/06-1772.1. JSTOR 27646105.
- Tedersoo L, Bahram M, Toots M, Diédhiou AG, Henkel TW, Kjøller R, Morris MH, Nara K, Nouhra E, Peay KG, Põlme S, Ryberg M, Smith ME, Kõljalg U (September 2012). "Towards global patterns in the diversity and community structure of ectomycorrhizal fungi". Molecular Ecology. 21 (17): 4160–70. doi:10.1111/j.1365-294X.2012.05602.x. PMID 22568722.
- van der Heijden MG, Bardgett RD, van Straalen NM (March 2008). "The unseen majority: soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems". Ecology Letters. 11 (3): 296–310. doi:10.1111/j.1461-0248.2007.01139.x. PMID 18047587.
- Marschner P, Crowley D, Rengel Z (2011). "Rhizosphere interactions between microorganisms and plants govern iron and phosphorus acquisition along the root axis – model and research methods". Soil Biology and Biochemistry. 43 (5): 883–94. doi:10.1016/j.soilbio.2011.01.005.
- Green LE, Porras-Alfaro A, Sinsabaugh RL (September 2008). "Translocation of Nitrogen and Carbon Integrates Biotic Crust and Grass Production in Desert Grassland". Journal of Ecology. 96 (5): 1076–85. doi:10.1111/j.1365-2745.2008.01388.x. JSTOR 20143553.
- Lugtenberg B, Kamilova F (2009). "Plant-growth-promoting rhizobacteria". Annual Review of Microbiology. 63: 541–56. doi:10.1146/annurev.micro.62.081307.162918. PMID 19575558.
- Latch, Garrick C.M. (1993). "Physiological interactions of endophytic fungi and their hosts. Biotic stress tolerance imparted to grasses by endophytes". Agriculture, Ecosystems & Environment. 44 (1–4): 143–56. doi:10.1016/0167-8809(93)90043-O.
- Rodriguez R, Redman R (2008). "More than 400 million years of evolution and some plants still can't make it on their own: plant stress tolerance via fungal symbiosis". Journal of Experimental Botany. 59 (5): 1109–14. doi:10.1093/jxb/erm342. PMID 18267941.
- Lau JA, Lennon JT (October 2011). "Evolutionary ecology of plant-microbe interactions: soil microbial structure alters selection on plant traits". The New Phytologist. 192 (1): 215–24. doi:10.1111/j.1469-8137.2011.03790.x. PMID 21658184.
- Porras-Alfaro A, Bayman P (2011). "Hidden fungi, emergent properties: endophytes and microbiomes". Annual Review of Phytopathology. 49: 291–315. doi:10.1146/annurev-phyto-080508-081831. PMID 19400639. S2CID 12770904.
- Doornbos RF, van Loon LC, Bakker PA (2011). "Impact of root exudates and plant defense signaling on bacterial communities in the rhizosphere. A review" (PDF). Agronomy for Sustainable Development. 32 (1): 227–43. doi:10.1007/s13593-011-0028-y. S2CID 26283073.
- Bakker AH, Doornbos RF, Zamioudis C, Berendsen RL, Pieterse CM (2013). "Induced Systemic Resistance and the Rhizosphere Microbiome". The Plant Pathology Journal. 29 (2): 136–43. doi:10.5423/PPJ.SI.07.2012.0111. PMC 4174772. PMID 25288940.
- Packer A, Clay K (March 2000). "Soil pathogens and spatial patterns of seedling mortality in a temperate tree". Nature. 404 (6775): 278–81. Bibcode:2000Natur.404..278P. doi:10.1038/35005072. PMID 10749209. S2CID 23292138.
- Smith SE, Read DJ (1996). Mycorrhizal Symbiosis (2nd ed.). New York, NY: Academic Press. ISBN 978-0-08-053719-1.
- Zeilinger S, Gupta VK, Dahms TE, Silva RN, Singh HB, Upadhyay RS, Gomes EV, Tsui CK, Nayak SC (March 2016). "Friends or foes? Emerging insights from fungal interactions with plants". FEMS Microbiology Reviews. 40 (2): 182–207. doi:10.1093/femsre/fuv045. PMC 4778271. PMID 26591004.
- Jumpponen A, Trappe JM (October 1998). "Dark Septate Endophytes: A Review of Facultative Biotrophic Root-Colonizing Fungi". New Phytologist. 140 (2): 295–310. doi:10.1046/j.1469-8137.1998.00265.x. JSTOR 2588371. PMID 33862835.
- Downie JA (March 2010). "The roles of extracellular proteins, polysaccharides and signals in the interactions of rhizobia with legume roots". FEMS Microbiology Reviews. 34 (2): 150–70. doi:10.1111/j.1574-6976.2009.00205.x. PMID 20070373.
- Wheatley RM, Poole PS (July 2018). "Mechanisms of bacterial attachment to roots". FEMS Microbiology Reviews. 42 (4): 448–461. doi:10.1093/femsre/fuy014. PMID 29672765.
- Udvardi M, Poole PS (2013-04-29). "Transport and metabolism in legume-rhizobia symbioses". Annual Review of Plant Biology. 64 (1): 781–805. doi:10.1146/annurev-arplant-050312-120235. PMID 23451778.
- Steenhoudt O, Vanderleyden J (October 2000). "Azospirillum, a free-living nitrogen-fixing bacterium closely associated with grasses: genetic, biochemical and ecological aspects". FEMS Microbiology Reviews. 24 (4): 487–506. doi:10.1111/j.1574-6976.2000.tb00552.x. PMID 10978548.
- Moissl-Eichinger, Christine; Pausan, Manuela; Taffner, Julian; Berg, Gabriele; Bang, Corinna; Schmitz, Ruth A. (2017-08-18). "Archaea Are Interactive Components of Complex Microbiomes". Trends in Microbiology. 26 (1): 70–85. doi:10.1016/j.tim.2017.07.004. ISSN 0966-842X. PMID 28826642.
- Chelius, M.K.; Triplett, E.W. (2001-02-23). "The Diversity of Archaea and Bacteria in Association with the Roots of Zea mays L." Microbial Ecology. 41 (3): 252–263. doi:10.1007/s002480000087. ISSN 0095-3628. PMID 11391463. S2CID 20069511.
- Prudence, Samuel M.M.; Worsley, Sarah; Balis, Lucas; Murrell, J. Colin; Lehtovirta-Morley, Laura; Hutchings, Matthew L. (2019-03-01). "Root-associated archaea: investigating the niche occupied by ammonia oxidising archaea within the wheat root microbiome". Access Microbiology. 1 (1A). doi:10.1099/acmi.ac2019.po0112. ISSN 2516-8290.
- Leininger, S.; Urich, T.; Schloter, M.; Schwark, L.; Qi, J.; Nicol, G. W.; Prosser, J. I.; Schuster, S. C.; Schleper, C. (2006-08-17). "Archaea predominate among ammonia-oxidizing prokaryotes in soils". Nature. 442 (7104): 806–809. Bibcode:2006Natur.442..806L. doi:10.1038/nature04983. ISSN 0028-0836. PMID 16915287. S2CID 4380804.
- Taffner, Julian; Erlacher, Armin; Bragina, Anastasia; Berg, Christian; Moissl-Eichinger, Christine; Berg, Gabriele (2018-05-09). "What Is the Role of Archaea in Plants? New Insights from the Vegetation of Alpine Bogs". mSphere. 3 (3). doi:10.1128/msphere.00122-18. ISSN 2379-5042. PMC 5956146. PMID 29743201.
- Pump, Judith; Pratscher, Jennifer; Conrad, Ralf (2015-01-27). "Colonization of rice roots with methanogenic archaea controls photosynthesis-derived methane emission". Environmental Microbiology. 17 (7): 2254–2260. doi:10.1111/1462-2920.12675. ISSN 1462-2912. PMID 25367104.
- Wang, Haitao; Su, Jianqiang; Zheng, Tianling; Yang, Xiaoru (2015-02-04). "Insights into the role of plant on ammonia-oxidizing bacteria and archaea in the mangrove ecosystem". Journal of Soils and Sediments. 15 (5): 1212–1223. doi:10.1007/s11368-015-1074-x. ISSN 1439-0108. S2CID 84753901.
- Herrmann, M.; Saunders, A. M.; Schramm, A. (2008-03-14). "Archaea Dominate the Ammonia-Oxidizing Community in the Rhizosphere of the Freshwater Macrophyte Littorella uniflora". Applied and Environmental Microbiology. 74 (10): 3279–3283. Bibcode:2008ApEnM..74.3279H. doi:10.1128/aem.02802-07. ISSN 0099-2240. PMC 2394948. PMID 18344332.
- Liu, Yin; Li, Hong; Liu, Qun Fang; Li, Yan Hong (2015-03-05). "Archaeal communities associated with roots of the common reed (Phragmites australis) in Beijing Cuihu Wetland". World Journal of Microbiology and Biotechnology. 31 (5): 823–832. doi:10.1007/s11274-015-1836-z. ISSN 0959-3993. PMID 25739566. S2CID 19039050.
- Chen, Xue-Ping; Zhu, Yong-Guan; Xia, Yue; Shen, Ju-Pei; He, Ji-Zheng (2008-07-08). "Ammonia-oxidizing archaea: important players in paddy rhizosphere soil?". Environmental Microbiology. 10 (8): 1978–1987. doi:10.1111/j.1462-2920.2008.01613.x. ISSN 1462-2912. PMID 18430011.
- Ke, Xiubin; Lu, Yahai; Conrad, Ralf (2013-08-28). "Different behaviour of methanogenic archaea and Thaumarchaeota in rice field microcosms". FEMS Microbiology Ecology. 87 (1): 18–29. doi:10.1111/1574-6941.12188. ISSN 0168-6496. PMID 23909555.
- Song, Geun Cheol; Im, Hyunjoo; Jung, Jihye; Lee, Soohyun; Jung, Man‐Young; Rhee, Sung‐Keun; Ryu, Choong‐Min (2019-01-21). "Plant growth‐promoting archaea trigger induced systemic resistance in Arabidopsis thaliana against Pectobacterium carotovorum and Pseudomonas syringae". Environmental Microbiology. 21 (3): 940–948. doi:10.1111/1462-2920.14486. ISSN 1462-2912. PMID 30461142. S2CID 53944018.
- Cheung, Man Kit; Wong, Chong Kim; Chu, Ka Hou; Kwan, Hoi Shan (2018-09-26). "Community Structure, Dynamics and Interactions of Bacteria, Archaea and Fungi in Subtropical Coastal Wetland Sediments". Scientific Reports. 8 (1): 14397. Bibcode:2018NatSR...814397C. doi:10.1038/s41598-018-32529-5. ISSN 2045-2322. PMC 6158284. PMID 30258074.
- Cadillo-Quiroz, Hinsby; Yavitt, Joseph B.; Zinder, Stephen H.; Thies, Janice E. (2009-12-22). "Diversity and Community Structure of Archaea Inhabiting the Rhizoplane of Two Contrasting Plants from an Acidic Bog". Microbial Ecology. 59 (4): 757–767. doi:10.1007/s00248-009-9628-3. ISSN 0095-3628. PMID 20024684. S2CID 22134029.
- Marschner P, Yang CH, Lieberei R, Crowley DE (2001). "Soil and plant specific effects on bacterial community composition in the rhizosphere". Soil Biology and Biochemistry. 33 (11): 1437–45. doi:10.1016/S0038-0717(01)00052-9.
- Broeckling CD, Broz AK, Bergelson J, Manter DK, Vivanco JM (February 2008). "Root exudates regulate soil fungal community composition and diversity". Applied and Environmental Microbiology. 74 (3): 738–44. Bibcode:2008ApEnM..74..738B. doi:10.1128/AEM.02188-07. PMC 2227741. PMID 18083870.
- Bogar LM, Kennedy PG (March 2013). "New wrinkles in an old paradigm: neighborhood effects can modify the structure and specificity of Alnus-associated ectomycorrhizal fungal communities". FEMS Microbiology Ecology. 83 (3): 767–77. doi:10.1111/1574-6941.12032. PMID 23078526.
- Meinhardt KA, Gehring CA (March 2012). "Disrupting mycorrhizal mutualisms: a potential mechanism by which exotic tamarisk outcompetes native cottonwoods". Ecological Applications. 22 (2): 532–49. doi:10.1890/11-1247.1. PMID 22611852.