Salivary gland tumour
Salivary gland tumours, also known as mucous gland adenomas[1] or neoplasms, are tumours that form in the tissues of salivary glands. The salivary glands are classified as major or minor. The major salivary glands consist of the parotid, submandibular, and sublingual glands. The minor salivary glands consist of 800 to 1000 small mucus-secreting glands located throughout the lining of the oral cavity.[2] Patients with these types of tumours may be asymptomatic.[1]
| Salivary gland tumour | |
|---|---|
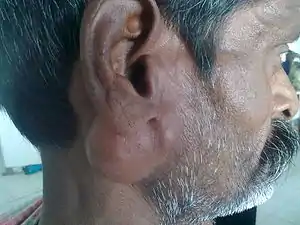 | |
| Parotid gland tumour | |
| Specialty | Oncology, oral and maxillofacial surgery, oral and maxillofacial pathology |
Presentation
Salivary gland tumours usually present as a lump or swelling in the affected gland which may or may not have been present for a long time. The lump may be accompanied by symptoms of duct blockage (e.g. xerostomia). Usually, in their early stages it is not possible to distinguish a benign tumour from a malignant one. One of the key differentiating symptoms of a malignant growth is nerve involvement; for example, signs of facial nerve damage (e.g. facial palsy) are associated with malignant parotid tumours. Facial pain and paraesthesia are also very often associated with malignant tumours.[3] Other red flag symptoms which may suggest malignancy and warrant further investigation are fixation of the lump to the overlying skin, ulceration and induration (hardening) of the mucosa.[4]
Diagnosis
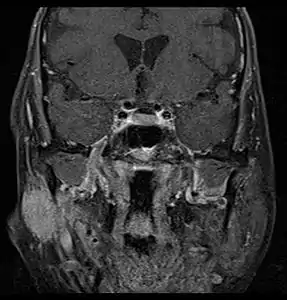
There are many diagnostic methods that can be used to determine the type of salivary gland tumour and if it is benign or malignant. Examples of diagnostic methods include:
Physical exam and history: An exam of the body to check general signs of health. The head, neck, mouth, and throat will be checked for signs of disease, such as lumps or anything else that seems unusual. A history of the patient's health habits and past illnesses and treatments will also be taken.
Endoscopy: A procedure to look at organs and tissues inside the body to check for abnormal areas. For salivary gland cancer, an endoscope is inserted into the mouth to look at the mouth, throat, and larynx. An endoscope is a thin, tube-like instrument with a light and a lens for viewing.
MRI or CT Scan: These tests can confirm the presence of a tumour. An MRI or CT Scan can also show whether metastasis has occurred.[5]
Biopsy: The removal of cells or tissues so they can be viewed under a microscope by a pathologist to check for signs of cancer.[6]
Fine needle aspiration (FNA) biopsy: The removal of tissue or fluid using a thin needle. An FNA is the most common type of biopsy used for salivary gland cancer, and has been shown to produce accurate results when differentiating between benign and malignant tumours.[7]
Radiographs: An OPG (orthopantomogram) can be taken to rule out mandibular involvement. A chest radiograph may also be taken to rule out any secondary tumours.[8]
Ultrasound: Ultrasound can be used to initially assess a tumour that is located superficially in either the submandibular or parotid gland. It can distinguish an intrinsic from an extrinsic neoplasm. Ultrasonic images of malignant tumours include ill-defined margins.[9] Furthermore, high resolution ultrasound can identify the exact tumour location within the parotid gland, its relationship to the retromandibular vein and assist surgical excision.[10]
Classification
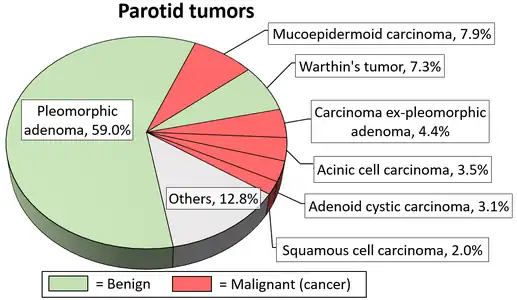

Due to the diverse nature of salivary gland tumours, many different terms and classification systems have been used. Perhaps the most widely used currently is that system proposed by the World Health Organization in 2005, which classifies salivary neoplasms as primary or secondary, benign or malignant, and also by tissue of origin. This system defines five broad categories of salivary gland neoplasms:[12][13]
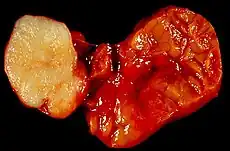
Benign epithelial tumors
- Pleomorphic adenoma
- Warthin's tumor
- Myoepithelioma
- Basal cell adenoma
- Oncocytoma
- Canalicular adenoma
- Lymphadenoma
- Sebaceous lymphadenoma
- Nonsebaceous lymphadenoma
- Ductal papilloma
- Inverted ductal papilloma
- Intraductal papilloma
- Sialadenoma papilliferum
- Cystadenoma
- Malignant epithelial tumors
- Acinic cell carcinoma
- Mucoepidermoid carcinoma
- Adenoid cystic carcinoma
- Polymorphous low-grade adenocarcinoma
- Epithelial-myoepithelial carcinoma
- Clear cell carcinoma, not otherwise specified
- Basal cell adenocarcinoma
- Sebaceous carcinoma
- Sebaceous lymphadenocarcinoma
- Cystadenocarcinoma
- Low-grade cribriform cystadenocarcinoma
- Mucinous adenocarcinoma
- Oncocytic carcinoma
- Salivary duct carcinoma
- Salivary duct carcinoma, not otherwise specified
- Adenocarcinoma, not otherwise specified
- Myoepithelial carcinoma
- Carcinoma ex pleomorphic adenoma
- Mammary analogue secretory carcinoma
- Carcinosarcoma
- Metastasizing pleomorphic adenoma
- Squamous cell carcinoma
- Large cell carcinoma
- Lymphoepithelial carcinoma
- Sialoblastoma
- Soft tissue tumors
- Hematolymphoid tumors
- Secondary tumors (i.e. a tumor which has metastasized to the salivary gland from a distant location)
Others, not included in the WHO classification above, include:[12]
- Intraosseous (central) salivary gland tumors
- Hybrid tumors (i.e. a tumor displaying combined forms of histologic tumor types)
- Hybrid carcinoma
- Others
- Others
- Keratocystoma
- Sialolipoma
Treatment
Most patients with early-stage lesions that are resectable generally tend to undergo surgery as their initial therapeutic approach, whereas those with advanced or unresectable cancers tend to be treated with radiotherapy (RT) alone or chemoradiotherapy (CRT), which hampered the comparison of the efficacy of RT alone with that of surgery combined with adjuvant RT. But some effort had been made to reflect the role of surgery in salivary gland tumours.

Treatment may include the following:
- Surgery Complete surgical resection, with adequate free margins, is currently the mainstay treatment for salivary gland tumours. However elective treatment of the N0 neck region remains a controversial topic
- Radiotherapy[5] If a salivary gland tumour is cancerous, Radiation Therapy may be necessary
Fast neutron therapy has been used successfully to treat salivary gland tumors,[14] and has shown to be significantly more effective than photons in studies treating unresectable salivary gland tumors.[15][16]
- Chemotherapy Currently little is known about the efficacy of chemotherapy in treating salivary gland tumours. Chemotherapy, which plays an important role in systemic therapy, is generally reserved for the palliative treatment of symptomatic locally recurrent and/or metastatic disease that is not amenable to further surgery or radiation. Conventional chemotherapy regimens, such as cisplatin and 5-FU or CAP (cisplatin, doxorubicin, and cyclophosphamide) are still utilized as first-line therapy for patients with advanced lesions.[17]
Targeted Therapy - Due to the poor results with chemotherapy, it's urgent to explore novel therapeutic interventions for this disease. And great expectations have been put into individualized therapies: in particular, the EGF receptors family (EGFR and HER2), KIT and androgen receptors are the most commonly investigated molecular targets in SGCs. Their expression seems not to be linked to its pathogenetic role in the development of SGCs, but more to the histogenetic origin of the tumor cells. Various targeted agents, such as imatinib, cetuximab, gefitinib, trastuzumab, had been used for exploring new treatment for salivary gland tumours, but on account of the rare incidence of salivary gland tumours, the number of cases available on targeted therapy for analysis is relatively small.[18]
Epidemiology
Little is known about the total incidence of salivary gland tumours as most benign tumours go unrecorded in national cancer registries.[3] The majority of salivary tumours are benign (65-70%).[4] Within the parotid gland 75 - 80% of tumours are benign. Around 50% of the tumours found in the submandibular glands are benign. Sublingual gland tumours are very rare but if present, they are most likely to be malignant.[4][19] Saku et al. in 1997 [20] and Venturi [21][22] in 2021, reported the causal role for ionizing radiation in salivary gland tumorigenesis, particularly for mucoepidermoid carcinoma.
In the United States, salivary gland cancers are uncommon with an incidence rate of 1.7 in 100000 between 2009 and 2013.[23]
References
- Zaleski, Michael P.; Kalhor, Neda; Moran, Cesar A. (November 2020). "Mucous Gland Adenoma: The Spectrum of Growth Patterns and the Diagnostic Challenges". Advances in Anatomic Pathology. 27 (6): 371–379. doi:10.1097/PAP.0000000000000283. ISSN 1072-4109. PMID 32909967. S2CID 221622196.
- Shah JP; Patel SG (2001). Cancer of the Head and Neck. PMPH-USA. p. 240. ISBN 978-1-55009-084-0.
- Odell, Edward W. (2017). Cawson's essentials of oral pathology and oral medicine (Ninth ed.). [Edinburgh]: Elsevier Health Sciences. ISBN 978-0702049828. OCLC 960030340.
- Mehanna, Hisham; McQueen, Andrew; Robinson, Max; Paleri, Vinidh (23 October 2012). "Salivary gland swellings". BMJ. 345: e6794. doi:10.1136/bmj.e6794. ISSN 1756-1833. PMID 23092898. S2CID 373247.
- "Salivary gland tumors: MedlinePlus Medical Encyclopedia". medlineplus.gov. Retrieved 28 October 2019.
- "Salivary Gland Cancer". MedicineNet.
- Vaishali H Anand et al. FNAC and Histopathology of Salivary Gland Tumors. SEAJCRR. 2014 Feb 3(1):609-618
- Mounika, C (7 March 2016). "Salivary Gland Tumors". SlideShare.
- Lee YY, Wong KT, King AD, Ahuja AT (June 2008). "Imaging of salivary gland tumours". Eur J Radiol. 66 (3): 419–36. doi:10.1016/j.ejrad.2008.01.027. PMID 18337041.
- Psychogios, Georgios; Rueger, Holger; Jering, Monika; Tsoures, Eleni; Künzel, Julian; Zenk, Johannes (September 2019). "Ultrasound can help to indirectly predict contact of parotid tumors to the facial nerve, correct intraglandular localization, and appropriate surgical technique". Head & Neck. 41 (9): 3211–3218. doi:10.1002/hed.25811. ISSN 1043-3074. PMID 31179604. S2CID 182948983.
- Steve C Lee, MD, PhD (22 December 2022). "Salivary Gland Neoplasms". Medscape.
{{cite journal}}: CS1 maint: multiple names: authors list (link) Updated: Jan 13, 2021
Diagrams by Mikael Häggström, MD - Barnes L (23 December 2008). Surgical Pathology of the Head and Neck. Vol. 1 (3rd ed.). Taylor & Francis. p. 511. ISBN 978-0-8493-9023-4.
- Barnes L (2005). "Chapter 5: Tumors of the salivary glands (chapter authors: Eveson JW, Auclair P, Gnepp DR, El-Naggar AK)" (PDF). Pathology and Genetics of Head and Neck Tumours. International Agency for Research on Cancer, World Health Organization. p. 210. ISBN 978-92-832-2417-4.
- Douglas JG, Koh WJ, Austin-Seymour M, Laramore GE (September 2003). "Treatment of salivary gland neoplasms with fast neutron radiotherapy". Arch. Otolaryngol. Head Neck Surg. 129 (9): 944–8. doi:10.1001/archotol.129.9.944. PMID 12975266.
- Laramore GE, Krall JM, Griffin TW, Duncan W, Richter MP, Saroja KR, Maor MH, Davis LW (September 1993). "Neutron versus photon irradiation for unresectable salivary gland tumors: final report of an RTOG-MRC randomized clinical trial. Radiation Therapy Oncology Group. Medical Research Council". Int. J. Radiat. Oncol. Biol. Phys. 27 (2): 235–40. doi:10.1016/0360-3016(93)90233-L. PMID 8407397.
- Krüll A, Schwarz R, Engenhart R, Huber P, Lessel A, Koppe H, Favre A, Breteau N, Auberger T (1996). "European results in neutron therapy of malignant salivary gland tumors". Bull Cancer Radiother. 83 Suppl: 125–9s. doi:10.1016/0924-4212(96)84897-3. PMID 8949764.
- Creagan, ET; Woods, JE; Schutt, AJ; O'Fallon, JR (1 December 1983). "Cyclophosphamide, adriamycin, and cis-diamminedichloroplatinum (II) in the treatment of advanced nonsquamous cell head and neck cancer". Cancer. 52 (11): 2007–10. doi:10.1002/1097-0142(19831201)52:11<2007::AID-CNCR2820521106>3.0.CO;2-T. PMID 6684986. S2CID 2813393.
- Mino M, Pilch BZ, Faquin WC (December 2003). "Expression of KIT (CD117) in neoplasms of the head and neck: an ancillary marker for adenoid cystic carcinoma". Mod. Pathol. 16 (12): 1224–31. doi:10.1097/01.MP.0000096046.42833.C7. PMID 14681323.
- "About salivary gland cancer | Salivary gland cancer | Cancer Research UK". www.cancerresearchuk.org. Retrieved 17 November 2017.
- Saku T, Hayashi Y, Takahara O, Matsuura H, Tokunaga M, Tokunaga M, Tokuoka S, Soda M, Mabuchi K, Land CE. (1997). "Salivary gland tumors among atomic bomb survivors, 1950-1987". Cancer. 15 (79 (8)): 1465–75. doi:10.1002/(SICI)1097-0142(19970415)79:8<1465::AID-CNCR4>3.0.CO;2-A. PMID 9118025. S2CID 11199713.
{{cite journal}}: CS1 maint: multiple names: authors list (link), - Venturi, Sebastiano (January 2021). "Cesium in Biology, Pancreatic Cancer, and Controversy in High and Low Radiation Exposure Damage—Scientific, Environmental, Geopolitical, and Economic Aspects". International Journal of Environmental Research and Public Health. 18 (17): 8934. doi:10.3390/ijerph18178934. PMC 8431133. PMID 34501532.
 Text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License. - Venturi, S. | Correlation of Diabetes, Salivary gland cancer and Pancreatic Cancer with Iodine and Cesium Radionuclides| Researchgate | December| 2022 | https:// www.researchgate.net/profile/Sebastiano-Venturi-4/publication/366530240
- American Cancer Society (2017). Cancer Facts and Figures 2017, Special Section: Rare Cancer in Adults. Atlanta: American Cancer Society.
External links
- Salivary gland cancer entry in the public domain NCI Dictionary of Cancer Terms
![]() This article incorporates public domain material from Dictionary of Cancer Terms. U.S. National Cancer Institute.
This article incorporates public domain material from Dictionary of Cancer Terms. U.S. National Cancer Institute.