Tofogliflozin
Tofogliflozin (INN,[1]: 88 USAN, codenamed CSG452) is an experimental drug for the treatment of diabetes mellitus and is being developed by Chugai Pharma in collaboration with Kowa and Sanofi.[2] It is an inhibitor of subtype 2 sodium-glucose transport protein (SGLT2), which is responsible for at least 90% of the glucose reabsorption in the kidney. As of September 2012, the drug is in Phase III clinical trials.[3][4]
 | |
| Clinical data | |
|---|---|
| Other names | CSG452 |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
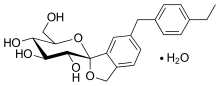
| Formula | C22H28O7 |
| Molar mass | 404.459 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Chemistry
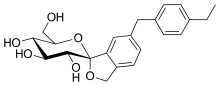
The active moiety or anhydrous form (ChemSpider ID: 28530778, CHEMBL2110731) has the chemical formula C22H26O6 and a molecular mass of 386.44 g/mol.
The United States Adopted Name tofogliflozin applies to the monohydrate, which is the form used as a drug.[5] The International Nonproprietary Name tofogliflozin applies to the anhydrous compound[1] and the drug form is referred to as tofogliflozin hydrate.
See also
References
- "International Nonproprietary Names for Pharmaceutical Substances (INN). Recommended International Nonproprietary Names: List 65" (PDF). World Health Organization. Retrieved 15 November 2016.
- Chugai Pharmaceutical: Development Pipeline
- Nagata T, Fukazawa M, Honda K, Yata T, Kawai M, Yamane M, et al. (February 2013). "Selective SGLT2 inhibition by tofogliflozin reduces renal glucose reabsorption under hyperglycemic but not under hypo- or euglycemic conditions in rats". American Journal of Physiology. Endocrinology and Metabolism. 304 (4): E414-23. doi:10.1152/ajpendo.00545.2012. PMID 23249697.
- Ohtake Y, Sato T, Kobayashi T, Nishimoto M, Taka N, Takano K, et al. (September 2012). "Discovery of tofogliflozin, a novel C-arylglucoside with an O-spiroketal ring system, as a highly selective sodium glucose cotransporter 2 (SGLT2) inhibitor for the treatment of type 2 diabetes". Journal of Medicinal Chemistry. 55 (17): 7828–40. doi:10.1021/jm300884k. PMID 22889351.
- Statement on a nonproprietary name adopted by the USAN council: Tofogliflozin.