Voglibose
Voglibose (INN and USAN, trade name Voglib, marketed by Mascot Health Series) is an alpha-glucosidase inhibitor used for lowering postprandial blood glucose levels in people with diabetes mellitus.[1] Voglibose delays the absorption of glucose thereby reducing the risk of macrovascular complications. Voglibose is a research product of Takeda Pharmaceutical Company, Japan's largest pharmaceutical company. Vogilbose was discovered in 1981, and was first launched in Japan in 1994,[2] under the trade name BASEN, to improve postprandial hyperglycemia in diabetes mellitus.[3]
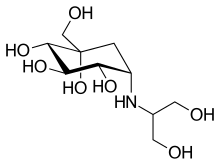 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C10H21NO7 |
| Molar mass | 267.278 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Postprandial hyperglycemia (PPHG) is primarily due to first phase insulin secretion. Alpha glucosidase inhibitors delay glucose absorption at the intestine level and thereby prevent sudden surge of glucose after a meal.[2]
There are three major drugs which belong to this class, acarbose, miglitol and voglibose,[2] of which voglibose is the newest.
Efficacy
A Cochrane systematic review assessed the effect of alpha-glucosidase inhibitors in people with impaired glucose tolerance, impaired fasting blood glucose, elevated glycated hemoglobin A1c (HbA1c).[4] It was found that there was no conclusive evidence that voglibose compared to diet and exercise or placebo reduced incidence of diabetes mellitus type 2, improved all-cause mortality, reduced or increased risk of cardiovascular mortality, serious or non-serious adverse events, non-fatal stroke, congestive heart failure, or non-fatal myocardial infarction.[4]
References
- Chen X, Zheng Y, Shen Y (2006). "Voglibose (Basen, AO-128), one of the most important alpha-glucosidase inhibitors". Current Medicinal Chemistry. 13 (1): 109–116. doi:10.2174/092986706789803035. PMID 16457643.
- Dabhi AS, Bhatt NR, Shah MJ (December 2013). "Voglibose: an alpha glucosidase inhibitor". Journal of Clinical and Diagnostic Research. 7 (12): 3023–3027. doi:10.7860/JCDR/2013/6373.3838. PMC 3919386. PMID 24551718.
- "Voglibose". AdisInsight. Springer Nature Switzerland AG.
- Moelands SV, Lucassen PL, Akkermans RP, De Grauw WJ, Van de Laar FA (December 2018). Cochrane Metabolic and Endocrine Disorders Group (ed.). "Alpha-glucosidase inhibitors for prevention or delay of type 2 diabetes mellitus and its associated complications in people at increased risk of developing type 2 diabetes mellitus". The Cochrane Database of Systematic Reviews. 2018 (12): CD005061. doi:10.1002/14651858.CD005061.pub3. PMC 6517235. PMID 30592787.
Further reading
- Miller CK (2004). "New therapeutic options in the treatment of diabetes mellitus". In Greenstein B (ed.). Clinical Pharmacology for nurses (17th ed.). Elsevier Limited, Churchill Livingstone.
- Wilson L (1997). Mehra IV (ed.). Managing the Patient with Type II Diabetes. Aspen Publishers. pp. 62–63. ISBN 978-0-8342-1018-9.