Undecaprenyl phosphate N,N'-diacetylbacillosamine 1-phosphate transferase
Phosphoglycosyl transferase C (PglC) is an enzyme belonging to a class known as monotopic phosphoglycosyl transferases (PGT). PGTs are required for the synthesis of glycoconjugates on the membrane surface of bacteria. Glycoconjugates, such as glycoproteins, are imperative for bacterial communication as well as host cell interactions between prokaryotic and eukaryotic cells lending to bacteria's pathogenicity.[1][2]
| Phosphoglycosyl transferase C (from Campylobacter jejuni) | |||||||
|---|---|---|---|---|---|---|---|
| Identifiers | |||||||
| Organism | |||||||
| Symbol | PglC | ||||||
| Entrez | 905415 | ||||||
| PDB | 5W7L | ||||||
| RefSeq (Prot) | WP_251831164.1 | ||||||
| UniProt | Q0P9D0 | ||||||
| |||||||
| Undecaprenyl phosphate N,N'-diacetylbacillosamine 1-phosphate transferase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| EC no. | 2.7.8.36 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| |||||||||
Background
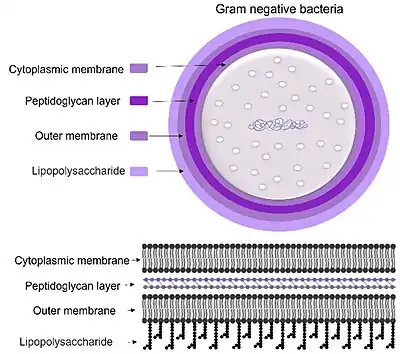
PglC is found in the pathogenic gram-negative organism Campylobacter jejuni (C. jejuni). Infection from C. jejuni results in acute gastroenteritis followed by vomiting, diarrhea, fever and abdominal pain.[3] The most common route of infection is through undercooked poultry as birds are a common source of C. jejuni.[4] Recent studies have also shown an association between prior C. jejuni infection and the neurological syndrome Guillan-Barré.[3][5][6] The glycoconjugates, lipopolysaccharides (LPS), found in the membrane of the bacteria resemble gangliosides found in the human nervous system leading to the generation of autoantibodies which cause deterioration of neurons.[5][6] Gangliosides can be found on neuronal cells and are membrane proteins that aid in cell-cell recognition and communication.
PglC belongs to a superfamily of enzymes known as monotopic phosphoglycsoyl transferases (monoPGT). These membrane-associated proteins catalyze the transfer of a phosphosugar from a soluble nucleoside diphosphate-activated donor to a polyprenol phosphate (Pren-P) acceptor within the membrane.[7] The product is then diversified via action of glycosyl transferases, to build a lipid linked oligosaccharide that will be flipped to the periplasm and form a glycoconjugate. Mono PGTs are unique to prokaryotes and essential for the production of glycoconjugates which mediate cell-host interactions during bacterial infections and are thus important for bacterial survival and pathogenicity.[7][8]
Glycoconjugates are integral structures on the surface of cell membranes composed of carbohydrates linked to other biomolecules such as proteins or lipids. These structures serve as a shield to the environment as well as aid in pathogenesis and viability of the bacterium itself. Glycoconjugates are also known to comprise adhesins used for host colonization and invasion.[1][9] C. jejuni utilizes adhesins to attach to the epithelial cells of the gastrointestinal tract of humans allowing for colonization and infection of the human host.[10][11][9] Eukaryotic cells exhibit their own glycoconjugate ecosystem lending to immune system recognition of human cells as "self". Bacteria utilize glycan mimicry to pose as eukaryotic cells and evade immune response.[1] Several studies have shown that inactivated genes linked to glycan synthesis result in an inability of bacteria to adhere to host cells thereby inhibiting colonization of the host.[2][12][13][14]
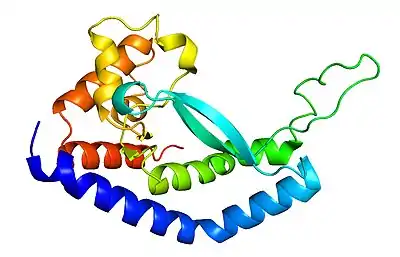
Structure
PglC is a membrane protein which only enters the first leaflet of the membrane on the cytosolic side of the lipid membrane interface. That is, the protein only sits in the first layer of the double-layered membrane. PglC from Campylobacter jejuni has yet to be structurally characterized, but an orthologue of PglC from Campylobacter concisus was elucidated in 2018 that represents the minimal functional core of this class of proteins.[8] Primary structure and hidden markov model computational analysis had actually predicted PglC to be a bitopic membrane protein. A bitopic membrane protein is one that passes through both layers of the membrane, but only does so once. However, structural characterization revealed that PglC only pans the first leaflet of the membrane. The structure also revealed significant characteristics of the protein important to its function such as the reentrant membrane helix (blue/light blue) that dips into the first leaflet of the membrane as well as the highly conserved Asp-Glu catalytic dyad within the active site (green loop).[8] The active site also holds a phosphate binding site and Mg2+ cofactor site important for coordinating reaction chemistry.[7][8] The other helices exist at the membrane interface (red, green, orange).
Enzyme Superfamily
Membrane proteins exist in three topologies: polytopic, bitopic, and monotopic, depending on the distribution of their domains throughout the membrane.[15] The domains of polytopic proteins cross the membrane multiple times while bitopic proteins may only pass through the membrane once typically with a transmembrane helix connecting two soluble domains outside of the membrane.[16][17] Monotopic membranes make up the smallest percentage of membrane proteins (0.06%) and are embedded in a single layer of the membrane, not both.[15] While the topologies of bitopic and polytopic membrane proteins can be linked to their function, monotopic proteins' topologies have yet to inform any function of these unique proteins apart from commonly being found in pathways where several enzymes are localized in sequence within the membrane.
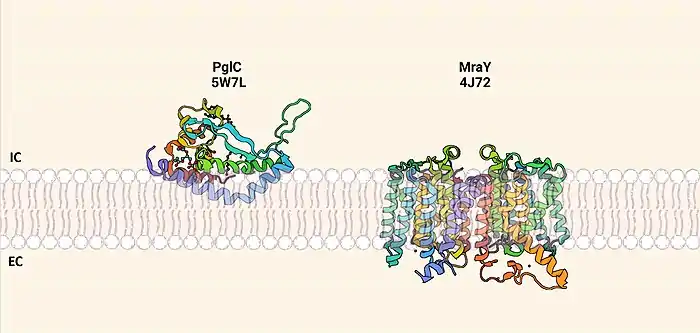
PglC is the first structurally characterized member of the monotopic PGT superfamily.[15][8] Other PGT superfamilies include the polytopic PGTs which are commonly exemplified by the proteins MraY and WecA. Although the three PGTs share the same function, they differ in structure and mechanism. PglC features a reentrant membrane helix that only spans the first leaflet of the membrane while MraY has multiple transmembrane helices. The mechanisms by which the two superfamilies' catalyze addition of an NDP-sugar (nucleoside di-phosphate) to a polyprenol acceptor within the membrane are unique. PglC utilizes a two-step ping pong mechanism that generates a covalent intermediate which is then available for nucleophilic attack by the polyprenol acceptor within the membrane.[7] MraY and other polytopic PGTs use a ternary complex mechanism whereby Pren-P and the NDP-sugar are reacted by enzyme within a single step.[18][19][7]
Function
PglC is involved in the first membrane-associated catalysis step involved in the synthesis of glycoconjugates in the bacterium Campylobacter jejuni. The substrate for PglC, UDP-di-N-acetyl-bacillosamine, is first synthesized in the cytosol by the enzymes PglD, PglE, and PglF.[20] PglC is responsible for linking the sugar, di-N-acetyl-bacillosamine, to the polyprenol phosphate (pren-p) acceptor within the membrane. PglC is also unique compared to its successors in the pathway due to the attachment of a phosphosugar to pren-p. PglA, PglJ, PglH, PglI, PglK, PglB are known as glycosyltransferases and each add their own respective sugars to the growing glycan, but there is no addition of a phosphoryl group. The phosphoryl group is integral for initiation of the membrane-associated part of the pathway, otherwise no successive sugars can be added to the growing glycan.
Mechanism
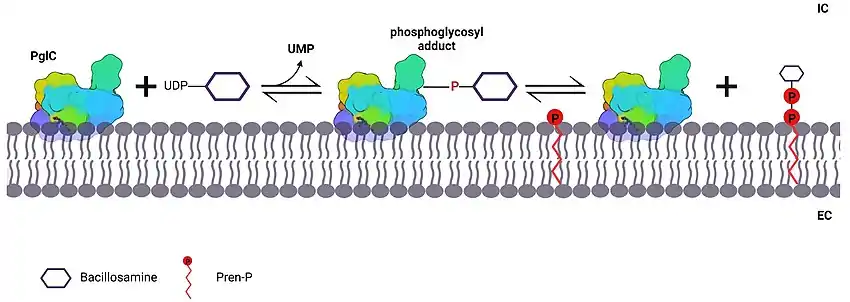
PglC catalyzes the generation of a polyprenol diphoshate-linked sugar (bacillosamine) via a ping pong mechanism.[7] The AspGlu catalytic dyad of PglC acts as a nucleophile to attack UDP-bacillosamine, releasing UMP in the process (step 1). A covalent intermediate characteristic of a ping pong mechanism is formed between the enzyme and the sugar via the phosphate group. Polyprenol phosphate (Pren-P), a membrane substrate, attacks the covalent intermediate, or phosphoglycosyl adduct, resulting in turnover of enzyme and attachment of the sugar to the Pren-P acceptor within the membrane.[7]
References
- Varki, Ajit; Gagneux, Pascal (2015), Varki, Ajit; Cummings, Richard D.; Esko, Jeffrey D.; Stanley, Pamela (eds.), "Biological Functions of Glycans", Essentials of Glycobiology (3rd ed.), Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press, doi:10.1101/glycobiology.3e.007 (inactive 1 August 2023), PMID 28876862, retrieved 2022-11-14
{{citation}}: CS1 maint: DOI inactive as of August 2023 (link) - Tra VN, Dube DH (May 2014). "Glycans in pathogenic bacteria--potential for targeted covalent therapeutics and imaging agents". Chemical Communications. 50 (36): 4659–4673. doi:10.1039/C4CC00660G. PMC 4049282. PMID 24647371.
- Perez-Perez, Guillermo I.; Blaser, Martin J. (1996), Baron, Samuel (ed.), "Campylobacter and Helicobacter", Medical Microbiology (4th ed.), Galveston (TX): University of Texas Medical Branch at Galveston, ISBN 978-0-9631172-1-2, PMID 21413331, retrieved 2022-11-20
- Allos BM (April 2001). "Campylobacter jejuni Infections: update on emerging issues and trends". Clinical Infectious Diseases. 32 (8): 1201–1206. doi:10.1086/319760. PMID 11283810.
- McCarthy N, Giesecke J (March 2001). "Incidence of Guillain-Barré syndrome following infection with Campylobacter jejuni". American Journal of Epidemiology. 153 (6): 610–614. doi:10.1093/aje/153.6.610. PMID 11257070.
- Nachamkin I, Allos BM, Ho T (July 1998). "Campylobacter species and Guillain-Barré syndrome". Clinical Microbiology Reviews. 11 (3): 555–567. doi:10.1128/CMR.11.3.555. PMC 88896. PMID 9665983.
- Das D, Kuzmic P, Imperiali B (July 2017). "Analysis of a dual domain phosphoglycosyl transferase reveals a ping-pong mechanism with a covalent enzyme intermediate". Proceedings of the National Academy of Sciences of the United States of America. 114 (27): 7019–7024. doi:10.1073/pnas.1703397114. PMC 5502628. PMID 28630348.
- Ray LC, Das D, Entova S, Lukose V, Lynch AJ, Imperiali B, Allen KN (June 2018). "Membrane association of monotopic phosphoglycosyl transferase underpins function". Nature Chemical Biology. 14 (6): 538–541. doi:10.1038/s41589-018-0054-z. PMC 6202225. PMID 29769739.
- Rubinchik S, Seddon A, Karlyshev AV (March 2012). "Molecular mechanisms and biological role of Campylobacter jejuni attachment to host cells". European Journal of Microbiology & Immunology. 2 (1): 32–40. doi:10.1556/EuJMI.2.2012.1.6. PMC 3933988. PMID 24611119.
- Fauchere JL, Rosenau A, Veron M, Moyen EN, Richard S, Pfister A (November 1986). "Association with HeLa cells of Campylobacter jejuni and Campylobacter coli isolated from human feces". Infection and Immunity. 54 (2): 283–287. doi:10.1128/iai.54.2.283-287.1986. PMC 260156. PMID 3770943.
- Janssen R, Krogfelt KA, Cawthraw SA, van Pelt W, Wagenaar JA, Owen RJ (July 2008). "Host-pathogen interactions in Campylobacter infections: the host perspective". Clinical Microbiology Reviews. 21 (3): 505–518. doi:10.1128/CMR.00055-07. PMC 2493085. PMID 18625685.
- Schirm M, Soo EC, Aubry AJ, Austin J, Thibault P, Logan SM (June 2003). "Structural, genetic and functional characterization of the flagellin glycosylation process in Helicobacter pylori". Molecular Microbiology. 48 (6): 1579–1592. doi:10.1046/j.1365-2958.2003.03527.x. PMID 12791140. S2CID 24095927.
- Smedley JG, Jewell E, Roguskie J, Horzempa J, Syboldt A, Stolz DB, Castric P (December 2005). "Influence of pilin glycosylation on Pseudomonas aeruginosa 1244 pilus function". Infection and Immunity. 73 (12): 7922–7931. doi:10.1128/iai.73.12.7922-7931.2005. PMC 1307089. PMID 16299283.
- Alemka A, Nothaft H, Zheng J, Szymanski CM (May 2013). "N-glycosylation of Campylobacter jejuni surface proteins promotes bacterial fitness". Infection and Immunity. 81 (5): 1674–1682. doi:10.1128/iai.01370-12. PMC 3648013. PMID 23460522.
- Allen KN, Entova S, Ray LC, Imperiali B (January 2019). "Monotopic Membrane Proteins Join the Fold". Trends in Biochemical Sciences. 44 (1): 7–20. doi:10.1016/j.tibs.2018.09.013. PMC 6309722. PMID 30337134.
- Lord JM, High S (March 2005). "Polytopic proteins: preventing aggregation in the membrane". Current Biology. 15 (5): R169–R171. doi:10.1016/j.cub.2005.02.043. PMID 15753028. S2CID 14404516.
- Zviling M, Kochva U, Arkin IT (March 2007). "How important are transmembrane helices of bitopic membrane proteins?". Biochimica et Biophysica Acta (BBA) - Biomembranes. 1768 (3): 387–392. doi:10.1016/j.bbamem.2006.11.019. PMID 17258687.
- O'Toole KH, Bernstein HM, Allen KN, Imperiali B (June 2021). "The surprising structural and mechanistic dichotomy of membrane-associated phosphoglycosyl transferases". Biochemical Society Transactions. 49 (3): 1189–1203. doi:10.1042/BST20200762. PMC 9206117. PMID 34100892.
- Al-Dabbagh B, Olatunji S, Crouvoisier M, El Ghachi M, Blanot D, Mengin-Lecreulx D, Bouhss A (August 2016). "Catalytic mechanism of MraY and WecA, two paralogues of the polyprenyl-phosphate N-acetylhexosamine 1-phosphate transferase superfamily". Biochimie. 127: 249–257. doi:10.1016/j.biochi.2016.06.005. PMID 27312048.
- Kumar M, Balaji PV (May 2011). "Comparative genomics analysis of completely sequenced microbial genomes reveals the ubiquity of N-linked glycosylation in prokaryotes". Molecular BioSystems. 7 (5): 1629–1645. doi:10.1039/c0mb00259c. PMID 21387023.
External links
- Undecaprenyl+phosphate+N,N'-diacetylbacillosamine+1-phosphate+transferase at the U.S. National Library of Medicine Medical Subject Headings (MeSH)