Vanadium nitride
Vanadium nitride, VN, is a chemical compound of vanadium and nitrogen.
 | |
| Names | |
|---|---|
| IUPAC name
Vanadium nitride | |
| Other names
Vanadium(III) nitride | |
| Identifiers | |
| ECHA InfoCard | 100.042.151 |
| EC Number |
|
PubChem CID |
|
| |
| Properties | |
| VN | |
| Molar mass | 64.9482 g/mol |
| Appearance | black powder |
| Density | 6.13 g/cm3 |
| Melting point | 2,050 °C (3,720 °F; 2,320 K) |
| Structure | |
| cubic, cF8 | |
| Fm3m, No. 225 | |
| Hazards | |
| GHS labelling: | |
 | |
| Warning | |
| H302, H312, H332 | |
| P261, P264, P270, P271, P280, P301+P312, P302+P352, P304+P312, P304+P340, P312, P322, P330, P363, P501 | |
| Flash point | Non-flammable |
| Related compounds | |
Other anions |
vanadium(III) oxide, vanadium carbide |
Other cations |
titanium nitride, chromium(III) nitride, niobium nitride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
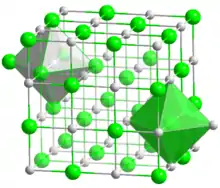
Vanadium nitride is formed during the nitriding of steel and increases wear resistance.[1] Another phase, V2N, also referred to as vanadium nitride, can be formed along with VN during nitriding.[2] VN has a cubic, rock-salt structure. There is also a low-temperature form, which contains V4 clusters.[3] The low-temperature phase results from a dynamic instability, when the energy of vibrational modes in the high-temperature NaCl-structure phase, are reduced below zero.[4]
It is a strong-coupled superconductor.[5] Nanocrystalline vanadium nitride has been claimed to have potential for use in supercapacitors.[6] The properties of vanadium nitride depend sensitively on the stoichiometry of the material.[7]
References
- Munozriofano, R; Casteletti, L; Nascente, P (2006). "Study of the wear behavior of ion nitrided steels with different vanadium contents". Surface and Coatings Technology. 200 (20–21): 6101. doi:10.1016/j.surfcoat.2005.09.026.
- Thermo reactive diffusion vanadium nitride coatings on AISI 1020 steel U.Sen Key Engineering Materials vols 264-268 (2004),577
- Kubel, F.; Lengauer, W.; Yvon, K.; Junod, A. (1988). "Structural phase transition at 205 K in stoichiometric vanadium nitride". Physical Review B. 38 (18): 12908. doi:10.1103/PhysRevB.38.12908.
- A. B. Mei; O. Hellman; N. Wireklint; C. M. Schlepütz; D. G. Sangiovanni; B. Alling; A. Rockett; L. Hultman; I. Petrov & J. E. Greene (2015). "Dynamic and structural stability of cubic vanadium nitride". Physical Review B. 91 (5): 054101. doi:10.1103/PhysRevB.91.054101.
- Zhao, B. R.; Chen, L.; Luo, H. L.; Mullin, D. P. (1984). "Superconducting and normal-state properties of vanadium nitride". Physical Review B. 29 (11): 6198. doi:10.1103/PhysRevB.29.6198.
- Choi, D.; Blomgren, G. E.; Kumta, P. N. (2006). "Fast and Reversible Surface Redox Reaction in Nanocrystalline Vanadium Nitride Supercapacitors". Advanced Materials. 18 (9): 1178. doi:10.1002/adma.200502471.
- Mei, A. B.; Tuteja, M.; Sangiovanni, D. G.; Haasch, R. T.; Rockett, A.; Hultman, L.; Petrov, I.; Greene, J. E. (2016-08-25). "Growth, nanostructure, and optical properties of epitaxial VNx/MgO(001) (0.80 ≤ x ≤ 1.00) layers deposited by reactive magnetron sputtering". Journal of Materials Chemistry C. 4 (34): 7924–7938. doi:10.1039/C6TC02289H. ISSN 2050-7534.