VCAM-1
Vascular cell adhesion protein 1 also known as vascular cell adhesion molecule 1 (VCAM-1) or cluster of differentiation 106 (CD106) is a protein that in humans is encoded by the VCAM1 gene.[5] VCAM-1 functions as a cell adhesion molecule.
Structure
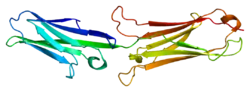
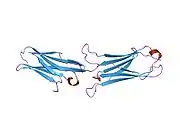
VCAM-1 is a member of the immunoglobulin superfamily, the superfamily of proteins including antibodies and T-cell receptors. The VCAM-1 gene contains six or seven immunoglobulin domains, and is expressed on both large and small blood vessels only after the endothelial cells are stimulated by cytokines. It is alternatively spliced into two known RNA transcripts that encode different isoforms in humans.[6] The gene product is a cell surface sialoglycoprotein, a type I membrane protein that is a member of the Ig superfamily.
Function
The VCAM-1 protein mediates the adhesion of lymphocytes, monocytes, eosinophils, and basophils to vascular endothelium. It also functions in leukocyte-endothelial cell signal transduction, and it may play a role in the development of atherosclerosis and rheumatoid arthritis.
Upregulation of VCAM-1 in endothelial cells by cytokines occurs as a result of increased gene transcription (e.g., in response to tumor necrosis factor-alpha (TNF-α) and interleukin-1 (IL-1)) and through stabilization of messenger RNA (mRNA) (e.g., Interleukin-4 (IL-4)). The promoter region of the VCAM1 gene contains functional tandem NF-κB (nuclear factor-kappa B) sites. The sustained expression of VCAM-1 lasts over 24 hours.
Primarily, the VCAM-1 protein is an endothelial ligand for VLA-4 (Very Late Antigen-4 or integrin α4β1) of the β1 subfamily of integrins. VCAM-1 expression has also been observed in other cell types (e.g., smooth muscle cells). It has also been shown to interact with EZR[7] and Moesin.[7]
VCAM-1 is also upregulated if vWF (Von Willebrand Factor) is given in knock-out (KO) ADMATS13 mice but not on mice without KO.[8]
CD106 also exists on the surface of some subpopulations of mesenchymal stem cells (MSC).[9]
Pharmacology
Certain melanoma cells can use VCAM-1 to adhere to the endothelium,[10] VCAM-1 may participate in monocyte recruitment to atherosclerotic sites, and it is highly overexpressed in the inflamed brain.[11] As a result, VCAM-1 is a potential drug target.
References
- GRCh38: Ensembl release 89: ENSG00000162692 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000027962 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
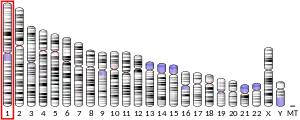
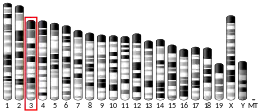
- Cybulsky M, Fries JW, Williams AJ, Sultan P, Eddy RL, Byers MG, Shows TB, Gimbrone MA Jr, Collins T (1991). "The human VCAM1 gene is assigned to chromosome 1p31-p32". Cytogenet. Cell Genet. 58 (3–4): 1850–1867. doi:10.1159/000133735.
- "Entrez Gene: VCAM1 vascular cell adhesion molecule 1".
- Barreiro O, Yanez-Mo M, Serrador JM, Montoya MC, Vicente-Manzanares M, Tejedor R, Furthmayr H, Sanchez-Madrid F (Jun 2002). "Dynamic interaction of VCAM-1 and ICAM-1 with moesin and ezrin in a novel endothelial docking structure for adherent leukocytes". J. Cell Biol. 157 (7): 1233–45. doi:10.1083/jcb.200112126. PMC 2173557. PMID 12082081.
- Le Besnerais M, Favre J, Denis CV, Mulder P, Martinet J, Nicol L, et al. (2016). "Assessment of endothelial damage and cardiac injury in a mouse model mimicking thrombotic thrombocytopenic purpura". J Thromb Haemost. 14 (10): 1917–1930. doi:10.1111/jth.13439. PMID 27501520.
- Yang ZX, Han ZB, Ji YR, Wang YW, Liang L, Chi Y, et al. (2013). "CD106 identifies a subpopulation of mesenchymal stem cells with unique immunomodulatory properties". PLOS One. 8 (3): e59354. Bibcode:2013PLoSO...859354Y. doi:10.1371/journal.pone.0059354. PMC 3595282. PMID 23555021.
- Eibl RH, Benoit M (2004). "Molecular resolution of cell adhesion forces". IEE Proceedings - Nanobiotechnology. 151 (3): 128–32. doi:10.1049/ip-nbt:20040707. PMID 16475855.
- Marcos-Contreras, Oscar A (2020). "Selective targeting of nanomedicine to inflamed cerebral vasculature to enhance the blood–brain barrier". Proceedings of the National Academy of Sciences of the United States of America. 117 (7): 3405–3414. doi:10.1073/pnas.1912012117. PMC 7035611. PMID 32005712.
Further reading
- Yonekawa K, Harlan JM (2005). "Targeting leukocyte integrins in human diseases". J. Leukoc. Biol. 77 (2): 129–40. doi:10.1189/jlb.0804460. PMID 15548573.
- Wu TC (2007). "The role of vascular cell adhesion molecule-1 in tumor immune evasion". Cancer Res. 67 (13): 6003–6. doi:10.1158/0008-5472.CAN-07-1543. PMC 3179385. PMID 17616653.