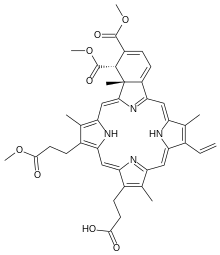
Verteporfin
Verteporfin (trade name Visudyne), a benzoporphyrin derivative, is a medication used as a photosensitizer for photodynamic therapy to eliminate the abnormal blood vessels in the eye associated with conditions such as the wet form of macular degeneration. Verteporfin accumulates in these abnormal blood vessels and, when stimulated by nonthermal red light with a wavelength of 689 nm[1] in the presence of oxygen, produces highly reactive short-lived singlet oxygen and other reactive oxygen radicals, resulting in local damage to the endothelium and blockage of the vessels.[2][3]
 | |
| Clinical data | |
|---|---|
| Trade names | Visudyne |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a607060 |
| License data | |
| Routes of administration | Intravenous |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C41H42N4O8 |
| Molar mass | 718.807 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Verteporfin is also used off-label for the treatment of central serous retinopathy.[4]
Administration
Verteporfin is usually injected intravenously into the largest arm vein.[5] It is injected at a dose of 6 mg/m2 and light-activated.[5] It is usually given 15 minutes before laser treatment.[2] This dose can be repeated 4 times per year.[5]
Side effects
Most common side effects are blurred vision, headache, and local effects at the injection site. Also, photosensitivity; it is strictly advised to avoid exposure to sunlight and unscreened lighting until 48 hours after verteporfin administration.[2]
Dogs and rats have been treated with inactivated daily doses 32–70 times higher than the dose advised for humans.[5] The 4 weeks of treatment resulted in mild extravascular hemolysis and hematopoiesis in the animals.[5]
Interactions
Verteporfin is known to interact with the herbal remedy feverfew (Tanacetum parthenium), the latter of which seems to act as an antagonist to verteporfin for unknown reasons. Taking the two substances simultaneously is inadvisable.[6]
Verteporfin does not appear to be metabolized by Cytochrome P450 enzymes, therefore not affecting Cytochrome P450 metabolism of other drugs.[7]
Shortages
In May 2020, a low manufacturing capacity caused disruption.[8] This affected the usage of verteporfin among providers and patients in Europe.[8] The EMA expected normal manufacturing to return by the first quarter 2022.[8]
Potential against scarring
Verteporfin is FDA approved.[9] Verteporfin is a YAP pathway inhibitor, interfering with its role in signaling following mechanic stress between cells and the extra-cellular matrix (Mechanotransduction).[10] Verteporfin displays a wide spectrum of anti-fibrotic properties.[5] Verteporfin prevents fibrosis in several human organs.[10] Verteporfin was first noted as a drug that blocked cell proliferation in the liver.[10] It is an inhibitor of fibrosis in patients with persistent cholestasis.[10] Research has highlighted that verteporfin decreased expression of fibrotic genes in fibroblasts collected from nodules of patients suffering from Dupuytren's contracture.[5] In 2018 information revealed verteporfin stopped fibrosis in the lung.[10] Verteporfin is a marketed drug with a good safety profile.[4] Physicians use verteporfin off-label.[4][8] In 2018, physicians described the off-label usage for Peyronie's disease as an interesting step.[5]
In 2021, scientists tested verteporfin to reveal if the drug would prevent scar tissue in skin.[11] The testing of verteporfin on humans cleft lips was due to occur in late 2021.[12]
References
- "Visudyne package insert" (PDF).
- Verteporfin Monograph
- Scott LJ, Goa KL (February 2000). "Verteporfin". Drugs & Aging. 16 (2): 139–46, discussion 147–8. doi:10.2165/00002512-200016020-00005. PMID 10755329. S2CID 260491296.
- Karim SP, Adelman RA (2013). "Profile of verteporfin and its potential for the treatment of central serous chorioretinopathy". Clinical Ophthalmology. 7: 1867–75. doi:10.2147/OPTH.S32177. PMC 3788817. PMID 24092965.
- Mohede, Daan C.J. (28 September 2018), "Verteporfin as a Medical Treatment in Peyronie's Disease", Sex Med, 6 (4): 302–308, doi:10.1016/j.esxm.2018.08.002, PMC 6302152, PMID 30274909
- "Feverfew and Verteporfin Interactions". Drugs.com. Retrieved 14 April 2015.
- "Visudyne (verteporfin for injection) prescribing information" (PDF). FDA. Retrieved 12 April 2021.
- EMA (5 November 2021). "Shortage of Visudyne (verteporfin)" (PDF). ema.europa.eu. Retrieved 1 February 2022.
- Mitash, N. (13 May 2022), "Inhibition of YAP/TAZ Signaling by the FDA Approved Drug Verteporfin Attenuates Fibrosis in Mouse and Human Tissue", Am J Respir Crit Care Med: A5226, doi:10.1164/ajrccm-conference.2022.205.1_MeetingAbstracts.A5226, S2CID 248633928
- Clark, Richard A. F. (29 July 2021), "To Scar or Not to Scar", N Engl J Med, 385 (5): 469–471, doi:10.1056/NEJMcibr2107204, PMID 34320296, S2CID 236498819
- Molteni, Megan, In mouse experiments, scientists unlock the key to scar-free skin healing, STAT News, April 22, 2021
- Kolata, Gina (22 April 2021). "Imagine, Surgery Without a Scar". nytimes,com. Retrieved 3 August 2021.
External links
- "Visudyne". Novartis.