Amniocentesis
Amniocentesis (also referred to as an amniotic fluid test or, informally, an "amnio") is a medical procedure[note 1] used primarily in prenatal diagnosis of chromosomal abnormalities and fetal infections[1] as well as for sex determination. In this procedure, a thin needle is inserted into the abdomen of the pregnant woman. The needle punctures the amnion, which is the membrane that surrounds the developing fetus. The fluid within the amnion is called amniotic fluid, and because this fluid surrounds the developing fetus, it contains fetal cells. The amniotic fluid is sampled, and the DNA within the collected fetal cells is tested for genetic abnormalities.
| Amniocentesis | |
|---|---|
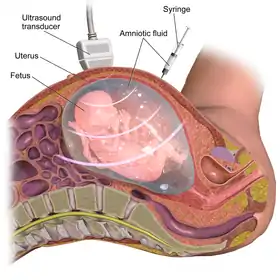 Amniocentesis | |
| Other names | Amniotic fluid test (AFT) |
| ICD-9-CM | 75.1 |
| MeSH | D000649 |
| MedlinePlus | 003921 |
The most common reason to have an amniocentesis performed is to determine whether a fetus has a genetic disorder, like a chromosomal abnormality (i.e., trisomy 21 which causes Down syndrome). Amniocentesis (or another procedure, called chorionic villus sampling (CVS)) can diagnose these problems in the womb.[2] These prenatal procedures can prove helpful to expectant guardians, as they allow for evaluating the fetal health status and the feasibility of treatment.[3]
An amniocentesis is performed when a woman is between 15 and 20 weeks gestation.[4] Women who choose to have this test are primarily those at increased risk for genetic and chromosomal problems, in part because the test is invasive and carries a small risk of miscarriage.[4] This process can be used for prenatal sex discernment and hence this procedure has legal restrictions in some countries.
Medical uses
Genetic diagnosis
Amniocentesis is offered to pregnant women whose fetus has an increased risk of chromosomal, genetic, and other fetal problems such as:[5][6]
- Down syndrome, also known as Trisomy 21
- Trisomy 13, also known as Patau syndrome
- Trisomy 18, also known as Edwards Syndome
- Sex chromosome aneuploidies, also known as sex chromosome anomalies
- Fragile X
- Neural tube defects (anencephaly and spina bifida) by alpha-fetoprotein levels.[7]
- Tay Sachs disease
- Rare, inherited metabolic disorders
Factors that increase the risk of a fetus developing these conditions and are indications for amniocentesis include family history of genetic disorders and a high risk cell-free fetal DNA screening result.[8]
Lung development
Amniocentesis can be used to test fetal lung development.[9] Problems with lung development can increase the risk of infant respiratory distress syndrome.[10] Fetal lung development can be tested by sampling the amount of surfactant in the amniotic fluid in pregnancies greater than 30 weeks. Several tests are available, including the lecithin-sphingomyelin ratio ("L/S ratio"), the presence of phosphatidylglycerol (PG), or the surfactant/albumin (S/A) ratio.
- For the L/S ratio, if the result is less than 2:1, the fetal lungs may be surfactant deficient.
- The presence of PG usually indicates fetal lung maturity.
- For the S/A ratio, the result is given as mg of surfactant per g of protein. An S/A ratio <35 indicates immature lungs, 35-55 is indeterminate, and >55 indicates mature surfactant production.
Infection
Amniocentesis can detect infections via decreased glucose level, a Gram stain showing bacteria, or abnormal differential count of white blood cells.[11] Common congenital infections during pregnancy that can be detected by amniocentesis include cytomegalovirus,[12] hepatitis B,[13] parvovirus B19,[14] and toxoplasmosis.[15] Early diagnosis of these infections facilitates the treatment of the pregnant woman with therapies such as immune globulins or antibiotics, leading to reduced sequelae in the fetus[15] or the possible prevention of maternal-fetal transmission.[13]
Rh Incompatibility
Amniocentesis can be used to diagnose Rh incompatibility, a condition when the mother has Rh-negative blood and the fetus has Rh-positive blood. Early detection is important to treat the mother with Rh immune globulin and to treat the fetus for hemolytic anemia.[16]
Decompression of polyhydramnios
Polyhydramnios, or the accumulation of amniotic fluids which leads to increase risk of cesarean section, can be relieved via decompression amniocentesis. Amniocentesis can also be used to diagnose potential causes of polyhydramnios.[17]
Preterm rupture of membranes
An emerging indication for amniocentesis is in the management of preterm rupture of membranes where measurement of certain amniotic fluid inflammatory markers may be helpful. If amniotic fluid IL-6, a marker of inflammation, is elevated, the fetus is at high risk and delivery should be considered.[18]
Contraindications
There are no absolute contraindications to amniocentesis. Relative contraindications to the procedure include failure to discontinue anticoagulation therapy 48-72 hours prior to amniocentesis, infections such as hepatitis B, hepatitis C, or human immunodeficiency virus (HIV), and decreased amniotic fluid volume for gestational age (oligohydramnios)[19]. These patients are at a higher risk of complications if they undergo amniocentesis and should be counseled appropriately. In some cases, the advantages of obtaining the results of an amniocentesis may outweigh the increased risk of complications.
Patients on oral anticoagulation therapy have an increased risk of bleeding from the procedure and may be switched to low-molecular-weight heparin, which carries a lower risk for bleeding complications, prior to amniocentesis.[19][20] There is an increased risk of mother-to-child (vertical) transmission of bloodborne infections in patients with hepatitis B, hepatitis C, or HIV after amniocentesis. Patients with high viral loads are at a greater risk of vertical transmission of hepatitis B compared to patients with low viral loads. In patients living with HIV, amniocentesis should be deferred until a combined antiretroviral therapy (CART) regimen is established and the patient achieves a low or undetectable viral load.[21]
While not a contraindication, an amniocentesis may be postponed if fusion of the amnion and chorion has not yet occurred. Performing an amniocentesis prior to the fusion of the amnion and chorion is more likely to lead to procedure failure that may require further sampling attempts.[21]
Risks/Complications
Amniocentesis is performed in the second trimester between the 15th and 20th weeks of pregnancy; performing this test in the first trimester is associated with increased risk of pregnancy loss and fetal congenital abnormalities.[22] The term "early amniocentesis" is used to describe use of the process between 9 to 14 weeks of gestation.[22]
Major risks associated with amniocentesis include pregnancy loss, vaginal bleeding or amniotic leakage after test, pre-labour ruptured membranes (aka "water breaking"), and preterm labor and delivery.[22] Additional potential complications of amniocentesis include, respiratory distress, postural deformities, chorioamnionitis, fetal trauma and alloimmunisation of the mother (rhesus disease). Studies from the 1970s originally estimated the risk of amniocentesis-related miscarriage at around 1 in 200 (0.5%).[23] Three more recent studies from 2000 to 2006 estimated the procedure-related pregnancy loss at 0.6-0.86%.[24][25] Unlike the previous studies, the number in this study only reflects the loss that resulted from amniocentesis complications and excluded the cases when parents decided for an abortion following the test results.[24] However, the most recent systematic analysis conducted in 2019 cites the procedure related risk of miscarriage associated with amniocentesis to be 0.30% (95%CI, 0.11-0.49%).[26]
Amniotic fluid embolism has also been described as a possible outcome.[27] Additional risks include amniotic fluid leakage and bleeding. These two are of particular importance because they can lead to spontaneous abortion in pregnant patients.[28]
While recognizing the aforementioned risks, the American College of Obstetricians and Gynecologists recommend that diagnostic testing options like amniocentesis be discussed with and offered to all patients that request them and that amniocentesis can be used after screening tests to confirm the presence of chromosomal abnormalities.[29]
The prenatal diagnosis of chromosomal abnormalities can have social drawbacks as technology changes the way people think about disability and kinship. One disadvantage associated with amniocentesis is that the results from the test are typically not available until after 17 weeks gestation.[22] This is late in pregnancy and can result in increased emotional distress for women who receive an unexpected fetal diagnosis.[22] In one sense, amniocentesis offers a window of control and in another, an anxiety-provoking responsibility to make rational decisions about complex, emotional and culturally contingent issues.[30][31]
Procedure/Technique
This procedure is typically performed in the outpatient setting by a team of providers including but not limited to an Obstetrics and Gynecology (OBGYN) physician, a nurse and an ultrasound technician. Typically at least two providers are needed for the procedure since hands must be used to hold the needle, hold the syringe and hold the ultrasound probe.[32] With transabdominal or transvaginal ultrasound guidance, a needle is inserted into the abdomen at an angle through the muscle, into the uterus and into the amniotic cavity.[33] The amniotic fluid is then aspirated and the fluid is analyzed for genetic abnormalities.[33] There are various methods to obtain samples including a single needle and double needle technique. These techniques have their own variations in how they are performed including guidance of needle insertion location, and angle of needle insertion.[34] The collected amniotic fluid is then submitted for laboratory testing for chromosomal abnormalities and the puncture site heals with time.[34]
Ultrasound evaluation
Before the procedure is performed, the fetus is analyzed via ultrasound to determine if it is viable and for any abnormalities. The ultrasound determines the location of the placenta, fetal position and movements, and characteristics of the amniotic fluid. This information is utilized to determine the type of needle used and how the procedure should be performed.[35]
Preparation
The abdomen is treated and cleaned with antiseptic before the procedure and/or the probe is covered with sterile coverings.[33] Sterile gel is also used on the abdomen before scanning with a sterile ultrasound probe. These measures are taken to reduce infection risk. The tools used in the procedure are coated with heparin to prevent clotting.[35]
Needle insertion
With the aid of ultrasound guidance, a needle is inserted through the mother's abdominal wall, then through the wall of the uterus, and finally into the amniotic sac. The physician then punctures the sac in an area away from the fetus and extracts approximately 20ml of amniotic fluid.[35] This procedure can be performed with a single needle or a double needle technique based on individualized patient factors and physician preference.[34] From the 20 ml of amniotic fluid, the first 2 ml is typically discarded due to mixture with maternal blood cells to ensure high quality fluid sampling.[19]
Post procedure analysis
If used for prenatal genetic diagnosis, fetal cells are separated by centrifugation from the extracted sample. The cells are grown in a culture medium, then fixed and stained. Under a microscope the chromosomes are examined for abnormalities. The most common abnormalities detected are Down syndrome (trisomy 21), Edwards syndrome (trisomy 18), and Turner syndrome (monosomy X). The sample is also analyzed for fetal infection and for intra-amniotic inflammation through infection studies.[35]
Recovery
After the procedure, the patient is instructed to take house rest for the initial 24 hours post-procedure however normal activities are allowed such as personal hygiene. In regard to the fetus, the puncture seals and the amniotic sac replenishes the liquid over the next 24–48 hours. One week after the procedure, the mother will have a follow up appointment for ultrasound analysis to confirm fetal viability and to assess healing of the puncture site.[35]
History
Tapping into amniotic fluid had become a common practice by the mid 1950s, as it was used to remove excess fluid from certain pregnancies with hydramnios.[36] Researchers then realized that they could use the fetal cells from the amniotic fluid to determine the chromosomal sex of the fetus. This was important to families with a history of X-linked diseases, because XY (male) children are more likely to inherit the disease than XX (female) children. In 1959, when French cytogeneticist Jerome LeJeune discovered that Down Syndrome is caused by trisomy 21, scientists realized the greater potential that amniocentesis has in providing information about many different types of chromosomal abnormalities.[36] By 1966, scientists were able to successfully culture fetal cells to perform a karyotype, which allowed them to visually see any large chromosomal abnormalities. Until the mid-1970s, amniocentesis procedures were done without the visual guidance from an ultrasound. In 1972, doctors Jens Bang and Allen Northeved from Denmark were the first to describe an amniocentesis done using an ultrasound.[37] Real-time ultrasound is now used during all amniocentesis procedures because it enhances the safety of the fetus.
Society and Culture
Prenatal sex discernment and sex-selective abortion
As stated in the history section, amniocentesis can be used to determine the sex of a fetus. This can be medically relevant in families that carry X-linked genetic conditions, since parents may want further genetic testing if the fetus is determined to be male (XY), and therefore has a higher likelihood of having the inherited disease. However, sex discernment is also used for social and cultural reasons. In some cultures, male children are more desirable than female children. This leads some parents to use amniocentesis and other forms of prenatal genetic testing (like chorionic villus sampling and preimplantation genetic diagnosis) to determine the sex of the child with the intent of terminating the pregnancy if the fetus is determined to have two X chromosomes. Sex-selective abortion is particularly common in countries such as China or India, among others. Sex-selective abortion is one of the causes for low child sex ratios in countries in Asia, Africa, and Eastern Europe. There are also significantly skewed child sex ratios in the Caucasus region. Naturally, the human sex ratio is approximately 105 males for every 100 females, and any significant deviations from these values is usually considered evidence for sex selective abortion.[38]
India and China have made prenatal sex determination illegal in an effort to prevent sex-selective abortion. In India, this happened through the 1994 Pre-Conception and Pre-Natal Diagnostic Techniques (Prohibition Of Sex Selection) Act (PCPNDT Act). However, this has not necessarily affected the widespread practice of sex-selective abortion as abortion is generally legal, and this law has been inconsistently enforced. In China, the societal preference for male children was exacerbated by the historical One-Child Policy, where in many regions of China, parents were limited to having only one child. As with India, prenatal sex determination is banned in China but remains a widespread practice, with enforcement also proving to be difficult.[39]
Stem cells
Amniotic fluid, which is easily collected, cultured, and stored through cryopreservation,[40] can be a rich source of pluripotent and multipotent mesenchymal, hematopoietic, neural, epithelial, and endothelial stem cells.[41][42][43]
A potential benefit of using amniotic stem cells over those obtained from embryos is that they address the ethical concerns among anti-abortion activists by obtaining pluripotent lines of undifferentiated cells without harm to a fetus or destruction of an embryo.[44] In addition, the use of embryonic cells has been shown to develop into tumors such as teratocarcinomas and frequently acquire chromosomal errors, underscoring the benefits of utilizing amniotic stem cells.[45] These stem cells would also, if used to treat the same individual they came from, sidestep the donor/recipient issue which has so far stymied all attempts to use donor-derived stem cells in therapies.
While significant research has shown that cells from second trimester amniotic fluid are successful at differentiating into various cell lines, third trimester cells, as well as amniotic fluid retrieved at the time of cesarean section at term,[46] have been shown to successfully differentiate into neural cell lineages with poorer differentiation into myocytes.[45]
Artificial heart valves, working tracheas, as well as muscle, fat, bone, heart, neural and liver cells have all been engineered through use of amniotic stem cells.[47] Tissues obtained from amniotic cell lines show significant promise for patients with congenital diseases/malformations of the heart, liver, lungs, kidneys, and cerebral tissue.[48][49]
The first amniotic stem cells bank in the US is active in Boston, Massachusetts.[50][51][52][53]
See also
- Chorionic villus sampling
- Amniotic fluid
- Amniotic stem cells
- Elective genetic and genomic testing
- Percutaneous umbilical cord blood sampling
- Prenatal diagnosis
Notes
- The word amniocentesis itself precisely indicates the procedure in question. The Greek word ἀμνίον amníon being the "inner membrane round the foetus," and κέντησις kéntēsis meaning "pricking," i.e. the puncture required in order to retrieve some amniotic fluid.
References
- "Diagnostic Tests – Amniocentesis". Harvard Medical School. Archived from the original on 2008-05-16. Retrieved 2008-07-15.
- Carlson LM, Vora NL (June 2017). "Prenatal Diagnosis: Screening and Diagnostic Tools". Obstetrics and Gynecology Clinics of North America. 44 (2): 245–256. doi:10.1016/j.ogc.2017.02.004. PMC 5548328. PMID 28499534.
- Cheng WL, Hsiao CH, Tseng HW, Lee TP (August 2015). "Noninvasive prenatal diagnosis". Taiwanese Journal of Obstetrics & Gynecology. 54 (4): 343–349. doi:10.1016/j.tjog.2015.05.002. PMID 26384048.
- "Amniocentesis (amniotic fluid test): MedlinePlus Medical Test". medlineplus.gov. Retrieved 2020-10-29.
- Prefumo F, Jauniaux E (January 2016). "Amniocentesis for fetal karyotyping: the end of an era?". BJOG. 123 (1): 99. doi:10.1111/1471-0528.13497. PMID 26715343.
- "Amniocentesis". nhs.uk. 2017-10-20. Retrieved 2022-09-14.
- Dungan JS, Elias S (November 2008). "Prenatal Diagnostic Testing". The Merck Manuals Online Medical Library. Archived from the original on 4 August 2010. Retrieved July 30, 2010.
- Wojcik MH, Reimers R, Poorvu T, Agrawal PB (July 2020). "Genetic diagnosis in the fetus". Journal of Perinatology. 40 (7): 997–1006. doi:10.1038/s41372-020-0627-z. PMC 7319864. PMID 32094481.
- "Amniocentesis (amniotic fluid test): MedlinePlus Medical Test". medlineplus.gov. Retrieved 2020-09-25.
- "Respiratory Distress Syndrome". www.nhlbi.nih.gov. Retrieved 2020-09-25.
- Medina TM, Hill DA (February 2006). "Preterm premature rupture of membranes: diagnosis and management". American Family Physician. 73 (4): 659–664. PMID 16506709.
- Leruez-Ville M, Foulon I, Pass R, Ville Y (September 2020). "Cytomegalovirus infection during pregnancy: state of the science". American Journal of Obstetrics and Gynecology. 223 (3): 330–349. doi:10.1016/j.ajog.2020.02.018. PMID 32105678. S2CID 211555165.
- Dionne-Odom J, Tita AT, Silverman NS (January 2016). "#38: Hepatitis B in pregnancy screening, treatment, and prevention of vertical transmission". American Journal of Obstetrics and Gynecology. 214 (1): 6–14. doi:10.1016/j.ajog.2015.09.100. PMID 26454123.
- Attwood LO, Holmes NE, Hui L (December 2020). "Identification and management of congenital parvovirus B19 infection". Prenatal Diagnosis. 40 (13): 1722–1731. doi:10.1002/pd.5819. hdl:11343/276375. PMID 32860469. S2CID 221365636.
- Martin S (June 2001). "Congenital toxoplasmosis". Neonatal Network. 20 (4): 23–30. doi:10.1891/0730-0832.20.4.23. PMID 12143899. S2CID 38109233.
- "Rh Incompatibility". www.nhlbi.nih.gov.
- Zeino S, Carbillon L, Pharisien I, Tigaizin A, Benchimol M, Murtada R, Boujenah J (April 2017). "Delivery outcomes of term pregnancy complicated by idiopathic polyhydramnios". Journal of Gynecology Obstetrics and Human Reproduction. 46 (4): 349–354. doi:10.1016/j.jogoh.2017.02.014. PMID 28643663.
- Kenyon AP, Abi-Nader KN, Pandya PP (2010). "Pre-Term Pre-Labour Rupture of Membranes and the Role of Amniocentesis". Fetal and Maternal Medicine Review. 21 (2): 75–88. doi:10.1017/S096553951000001X.
- Jindal, A.; Sharma, M.; Chaudhary, C. (2020). "Amniocentesis". StatPearls. PMID 32644673. Retrieved 19 October 2020.
- Sandercock PA, Leong TS, et al. (Cochrane Stroke Group) (April 2017). "Low-molecular-weight heparins or heparinoids versus standard unfractionated heparin for acute ischaemic stroke". The Cochrane Database of Systematic Reviews. 4 (4): CD000119. doi:10.1002/14651858.CD000119.pub4. PMC 6478133. PMID 28374884.
- "Practice Bulletin No. 162: Prenatal Diagnostic Testing for Genetic Disorders". Obstetrics & Gynecology. 127 (5): e108–e122. May 2016. doi:10.1097/AOG.0000000000001405. ISSN 0029-7844.
- Alfirevic Z, Navaratnam K, Mujezinovic F, et al. (Cochrane Pregnancy and Childbirth Group) (September 2017). "Amniocentesis and chorionic villus sampling for prenatal diagnosis". The Cochrane Database of Systematic Reviews. 2017 (9): CD003252. doi:10.1002/14651858.CD003252.pub2. PMC 6483702. PMID 28869276.
- Hitti M (1 November 2006). Chang L (ed.). "Amniocentesis Risk Overrated?". Webmd.com. Retrieved 12 November 2011.
- Wilson RD, Langlois S, Johnson JA (July 2007). "Mid-trimester amniocentesis fetal loss rate". Journal of Obstetrics and Gynaecology Canada. 29 (7): 586–590. doi:10.1016/S1701-2163(16)32501-4. PMID 17623573.
- Eddleman KA, Malone FD, Sullivan L, Dukes K, Berkowitz RL, Kharbutli Y, et al. (November 2006). "Pregnancy loss rates after midtrimester amniocentesis". Obstetrics and Gynecology. 108 (5): 1067–1072. doi:10.1097/01.AOG.0000240135.13594.07. PMID 17077226. S2CID 19081825.
- Salomon LJ, Sotiriadis A, Wulff CB, Odibo A, Akolekar R (October 2019). "Risk of miscarriage following amniocentesis or chorionic villus sampling: systematic review of literature and updated meta-analysis". Ultrasound in Obstetrics & Gynecology. 54 (4): 442–451. doi:10.1002/uog.20353. PMID 31124209. S2CID 163168333.
- Dodgson J, Martin J, Boswell J, Goodall HB, Smith R (May 1987). "Probable amniotic fluid embolism precipitated by amniocentesis and treated by exchange transfusion". British Medical Journal. 294 (6583): 1322–1323. doi:10.1136/bmj.294.6583.1322. PMC 1246486. PMID 3109636.
- Tara F, Lotfalizadeh M, Moeindarbari S (August 2016). "The effect of diagnostic amniocentesis and its complications on early spontaneous abortion". Electronic Physician. 8 (8): 2787–2792. doi:10.19082/2787. PMC 5053461. PMID 27757190.
- American College of Obstetricians Gynecologists' Committee on Practice Bulletins—Obstetrics; Committee on Genetics; Society for Maternal-Fetal Medicine (October 2020). "Screening for Fetal Chromosomal Abnormalities: ACOG Practice Bulletin, Number 226". Obstetrics and Gynecology. 136 (4): e48–e69. doi:10.1097/AOG.0000000000004084. PMID 32804883. S2CID 38007206.
- Lock M, Nguyen V (2010). An Anthropology of Biomedicine. Oxford: Wiley-Blackwell.
- Rapp R (1998). "Refusing prenatal diagnosis: the meanings of bioscience in a multicultural world". Science, Technology & Human Values. 23 (1): 45–70. doi:10.1177/016224399802300103. PMID 11660551. S2CID 45990911.
- Nizard J (April 2010). "Amniocentesis: technique and education". Current Opinion in Obstetrics & Gynecology. 22 (2): 152–154. doi:10.1097/GCO.0b013e32833723a0. PMID 20098324. S2CID 2905206.
- Mujezinovic F, Alfirevic Z, et al. (Cochrane Pregnancy and Childbirth Group) (August 2012). "Technique modifications for reducing the risks from amniocentesis or chorionic villus sampling". The Cochrane Database of Systematic Reviews (8): CD008678. doi:10.1002/14651858.CD008678.pub2. PMID 22895978.
- Monni G, Pagani G, Stagnati V, Iuculano A, Ibba RM (2016-05-02). "How to perform transabdominal chorionic villus sampling: a practical guideline". The Journal of Maternal-Fetal & Neonatal Medicine. 29 (9): 1499–1505. doi:10.3109/14767058.2015.1051959. PMID 26372474. S2CID 32311634.
- Cruz-Lemini M, Parra-Saavedra M, Borobio V, Bennasar M, Goncé A, Martínez JM, Borrell A (December 2014). "How to perform an amniocentesis". Ultrasound in Obstetrics & Gynecology. 44 (6): 727–731. doi:10.1002/uog.14680. PMID 25449117. S2CID 30283309.
- Cowan RS (April 1993). "Aspects of the history of prenatal diagnosis". Fetal Diagnosis and Therapy. 8 (Suppl. 1): 10–17. doi:10.1159/000263869. PMID 11653011.
- Bang J, Northeved A (November 1972). "A new ultrasonic method for transabdominal amniocentesis". American Journal of Obstetrics and Gynecology. 114 (5): 599–601. doi:10.1016/0002-9378(72)90835-6. PMID 4637046.
- Bowman-Smart H, Savulescu J, Gyngell C, Mand C, Delatycki MB (March 2020). "Sex selection and non-invasive prenatal testing: A review of current practices, evidence, and ethical issues". Prenatal Diagnosis. 40 (4): 398–407. doi:10.1002/pd.5555. PMC 7187249. PMID 31499588.
- Bowman-Smart H, Savulescu J, Gyngell C, Mand C, Delatycki MB (March 2020). "Sex selection and non-invasive prenatal testing: A review of current practices, evidence, and ethical issues". Prenatal Diagnosis. 40 (4): 398–407. doi:10.1002/pd.5555. PMC 7187249. PMID 31499588.
- Dziadosz M, Basch RS, Young BK (March 2016). "Human amniotic fluid: a source of stem cells for possible therapeutic use". American Journal of Obstetrics and Gynecology. 214 (3): 321–327. doi:10.1016/j.ajog.2015.12.061. PMID 26767797.
- Weiss R (2007-01-08). "Scientists See Potential In Amniotic Stem Cells". The Washington Post. Retrieved 2010-04-23.
- De Coppi P, Bartsch G, Siddiqui MM, Xu T, Santos CC, Perin L, et al. (January 2007). "Isolation of amniotic stem cell lines with potential for therapy". Nature Biotechnology. 25 (1): 100–106. doi:10.1038/nbt1274. PMID 17206138. S2CID 6676167.
- "Stem Cells – BiocellCenter". Archived from the original on 11 January 2010. Retrieved 2010-01-11.
- Lo B, Parham L (May 2009). "Ethical issues in stem cell research". Endocrine Reviews. 30 (3): 204–213. doi:10.1210/er.2008-0031. PMC 2726839. PMID 19366754.
- Dziadosz M, Basch RS, Young BK (March 2016). "Human amniotic fluid: a source of stem cells for possible therapeutic use". American Journal of Obstetrics and Gynecology. 214 (3): 321–327. doi:10.1016/j.ajog.2015.12.061. PMID 26767797.
- You Q, Tong X, Guan Y, Zhang D, Huang M, Zhang Y, Zheng J (February 2009). "The biological characteristics of human third trimester amniotic fluid stem cells". The Journal of International Medical Research. 37 (1): 105–112. doi:10.1177/147323000903700112. PMID 19215679. S2CID 27073963.
- Bajek A, Olkowska J, Gurtowska N, Kloskowski T, Walentowicz-Sadlecka M, Sadlecki P, et al. (June 2014). "Human amniotic-fluid-derived stem cells: a unique source for regenerative medicine". Expert Opinion on Biological Therapy. 14 (6): 831–839. doi:10.1517/14712598.2014.898749. PMID 24655038. S2CID 33654830.
- "Stem cells scientific updates – BiocellCenter". Archived from the original on 11 January 2010. Retrieved 2010-01-11.
- Petsche Connell J, Camci-Unal G, Khademhosseini A, Jacot JG (August 2013). "Amniotic fluid-derived stem cells for cardiovascular tissue engineering applications". Tissue Engineering. Part B, Reviews. 19 (4): 368–379. doi:10.1089/ten.teb.2012.0561. PMC 3690092. PMID 23350771.
- "European Biotech Company Biocell Center Opens First U.S. Facility for Preservation of Amniotic Stem Cells in Medford, Massachusetts". Reuters. 2009-10-22. Archived from the original on October 30, 2009. Retrieved 2010-01-11.
- "Europe's Biocell Center opens Medford office – Daily Business Update – The Boston Globe". 2009-10-22. Archived from the original on 12 January 2010. Retrieved 2010-01-11.
- "The Ticker - BostonHerald.com". Archived from the original on 2012-09-21. Retrieved 2010-01-11.
- "Biocell partner with largest New England's hospital group to preserve amniotic stem cell". Archived from the original on 14 March 2010. Retrieved 2010-03-10.
External links
 Media related to Amniocentesis at Wikimedia Commons
Media related to Amniocentesis at Wikimedia Commons- Amniodex is an interactive decision support intervention designed for women faced with the decision of whether to undergo amniocentesis.
- The Amniocentesis Report Archived 2012-03-01 at the Wayback Machine A Decision Guide for Expectant Parents and Health Care Professionals