Angiotensin II (medication)
Angiotensin II (Ang II) is a medication that is used to treat hypotension resulting from septic shock or other distributive shock. It is a synthetic vasoconstrictor peptide that is identical to human hormone angiotensin II[1] and is marketed under the brand name Giapreza. The Food and Drug Administration approved the use of angiotensin II in December 2017 to treat low blood pressure resulting from septic shock.[2]
 | |
| Clinical data | |
|---|---|
| Trade names | Giapreza |
| AHFS/Drugs.com | Monograph |
| Routes of administration | Intravenous injection |
| Drug class | Vasoconstrictor |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | None |
| Metabolism | Proteolysis by glutamyl aminopeptidase, angiotensin converting enzyme 2 |
| Metabolites | Angiotensin III, angiotensin-(1-7) |
| Elimination half-life | Less than one minute (IV administration) |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
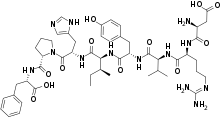
| Formula | C50H71N13O12 |
| Molar mass | 1046.197 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
The U.S. Food and Drug Administration (FDA) considers it to be a first-in-class medication.[3]
Medical uses
Angiotensin II is a vasoconstrictor used to increase blood pressure in adults with septic or other distributive shock. Angiotensin II is a naturally occurring hormone secreted as part of the renin-angiotensin system that results in powerful systemic vasoconstriction.[4][5] The vasopressor effects of angiotensin have been studied since it was first isolated in the late 1930s.[6] Vasopressors are defined as agents that combat vasodilatory shock by inducing peripheral vasoconstriction. Commonly used vasopressors include catecholamine (e.g., dopamine, norepinephrine, epinephrine) and non-catecholamine (e.g., vasopressin). but these agents are not always effective in reversing vasodilatory shock, and their use can be associated with significant side effects including limb ischemia and cardiac arrhythmia. Angiotensin II is as a treatment option that can increase blood pressure and allow catecholamine dose reductions.
Angiotensin II must be administered as an intravenous infusion diluted in 0.9% sodium chloride prior to use. The recommended starting dosage of angiotensin II is 20 nanograms (ng)/kg/min via continuous intravenous infusion. Administration through a central venous line is recommended. Monitor blood pressure response and titrate angiotensin II every 5 minutes by increments of up to 15 ng/kg/min as needed to achieve or maintain target blood pressure.[7]
Adverse effects
Angiotensin II treated patients are at an increased risk of thromboembolic events. There was a higher incidence of arterial and venous thrombotic and thromboembolic events in patients who received angiotensin II compared to placebo treated patients in the ATHOS-3 study [13% (21/163 patients) vs. 5% (8/158 patients)].[8] It is recommended that patients be on concurrent venous thromboembolism prophylaxis. Other adverse reactions include thrombocytopenia, tachycardia, fungal infection, delirium, acidosis, hyperglycemia, and peripheral ischemia.[7]
Angiotensin II acts on Angiotensin receptor (AT1) on presynaptic adrenergic nerves → release of catecholamine → excessive catecholamine can be harmful as it can cause myocyte necrosis.[9]
Interactions
Angiotensin II receptor blockers, as their name implies, block the action of angiotensin II at its receptors and therefore may reduce its effects.
References
- Kaufman MB (March 2018). "Pharmaceutical Approval Update". P & T. 43 (3): 141–170. PMC 5821238. PMID 29491694.
- "FDA approves drug to treat dangerously low blood pressure" (Press release). U.S. Food and Drug Administration (FDA). 21 December 2017.
- New Drug Therapy Approvals 2017 (PDF). U.S. Food and Drug Administration (FDA) (Report). January 2018. Retrieved 16 September 2020.
- Brown SM, Lanspa MJ, Jones JP, Kuttler KG, Li Y, Carlson R, Miller RR, Hirshberg EL, Grissom CK, Morris AH (March 2013). "Survival after shock requiring high-dose vasopressor therapy". Chest. 143 (3): 664–671. doi:10.1378/chest.12-1106. PMC 3590882. PMID 22911566.
- Chawla LS, Busse L, Brasha-Mitchell E, Davison D, Honiq J, Alotaibi Z, Seneff MG (October 2014). "Intravenous angiotensin II for the treatment of high-output shock (ATHOS trial): a pilot study". Critical Care. 18 (5): 534. doi:10.1186/s13054-014-0534-9. PMC 4212099. PMID 25286986.
- Bradley SE, Parker B (November 1941). "The Hemodynamic Effects of Angiotonin in Normal Man 1". The Journal of Clinical Investigation. 20 (6): 715–9. doi:10.1172/JCI101265. PMC 435102. PMID 16694877.
- "Giapreza- angiotensin ii injection". DailyMed. 20 June 2020. Retrieved 17 September 2020.
- Khanna A, English SW, Wang XS, Ham K, Tumlin J, Szerlip H, et al. (August 2017). "Angiotensin II for the Treatment of Vasodilatory Shock" (PDF). The New England Journal of Medicine. 377 (5): 419–430. doi:10.1056/NEJMoa1704154. PMID 28528561. S2CID 205102054.
- Liaudet, Lucas; Calderari, Belinda; Pacher, Pal (2014-11-01). "Pathophysiological mechanisms of catecholamine and cocaine-mediated cardiotoxicity" (PDF). Heart Failure Reviews. 19 (6): 815–824. doi:10.1007/s10741-014-9418-y. ISSN 1573-7322. PMID 24398587. S2CID 22420796.
External links
- "Angiotensin II". Drug Information Portal. U.S. National Library of Medicine.
- "Angiotensin II acetate". Drug Information Portal. U.S. National Library of Medicine.