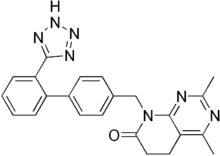
Tasosartan
Tasosartan is an angiotensin II receptor antagonist.
 | |
| Clinical data | |
|---|---|
| Pregnancy category |
|
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C23H21N7O |
| Molar mass | 411.469 g·mol−1 |
| | |
It was withdrawn from FDA review by the manufacturer after phase III clinical trials showed elevated transaminases (a sign of possible liver toxicity) in a significant number of participants given the drug.[1][2]
References
- Atkinson AJ, et al. (2007). Principles of clinical pharmacology. Amsterdam: Elsevier. p. 515. ISBN 978-0-12-369417-1.
- Dina R, Jafari M (July 2000). "Angiotensin II-receptor antagonists: an overview". Am J Health Syst Pharm. 57 (13): 1231–41. doi:10.1093/ajhp/57.13.1231. PMID 10902066.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.